Arthrotek tablets 75mg+200mcg 30 pcs. — Instructions
Release form
Tablets, 30 pieces per pack.
Compound
1 tablet contains the active ingredients: diclofenac sodium (50 mg), misoprostol (75 mg).
Additional components: aerosil, methylhydroxypropylcellulose, microcrystalline cellulose, crospovidone, corn starch, hydrogenated castor oil, lactose, cellulose acetate phthalate, magnesium stearate, diethyl phthalate and povidone.
Pharmacological properties
The therapeutic effect of Arthrotek is based on a combination of the active ingredients diclofenac and misoprostol.
The anti-inflammatory and analgesic properties of diclofenac are combined with the protective effect of misoprostol, which reliably protects the gastric mucosa from the negative effects of diclofenac.
Indications for use
Arthrotek is used and prescribed for acute joint-muscular pain syndrome, ankylosing spondylitis, osteoarthritis, rheumatoid arthritis and rheumatic diseases.
Directions for use and dosage
The tablets should be taken during meals, whole, without crushing or chewing, and with a sufficient amount of water.
The recommended dose is 1 tablet 2-3 times a day.
The duration of treatment is determined by the doctor individually for each patient.
Contraindications
The use of the drug Arthrotek is contraindicated:
- individual intolerance to the components included in the composition;
- gastrointestinal bleeding;
- aspirin bronchial asthma.
For diseases such as anemia, arterial hypertension, congestive heart failure, erosive and ulcerative pathology of the gastrointestinal tract, severe kidney and liver diseases, porphyria, dehydration and fluid retention in the body, the drug is taken with extreme caution.
Pregnancy and breastfeeding period
Pregnancy and breastfeeding are contraindications for the use of the drug Arthrotek.
Overdose
An overdose of diclofenac, which is part of the drug, causes: abdominal pain, confusion, drowsiness, bleeding and decreased muscle tone.
Symptoms of misoprostol poisoning are convulsions, shortness of breath, tremors, cardiac arrhythmias, abdominal pain, diarrhea, hypotension, fever.
In case of drug poisoning, gastric lavage should be performed, enterosobents should be taken and symptomatic treatment should be applied.
Adverse reactions
The use of the drug Arthrotek can cause adverse reactions from various organs and systems, namely:
gastrointestinal tract: diarrhea, constipation, abdominal pain, duodenitis, dyspepsia, esophagitis, belching, vomiting and nausea, flatulence, gastritis, gastrointestinal perforation or bleeding. allergic reactions: skin itching, hemorrhagic rash, bullous rashes, angioedema, anaphylaxis, epidermal necrolysis.
Others: headaches, insomnia, dizziness, hepatitis, interstitial nephritis, pancreatitis, anemia, acute renal failure, thrombocytopenia, difficult dreams, mood changes, blurred vision.
In women, manifestations from the mammary glands and reproductive system are possible: bleeding between menstruation, pain in the mammary glands, dysmenorrhea, menorrhagia, pain in the lower abdomen of a cramping nature, the menstrual cycle is disrupted, vaginitis, spotting in postmenopause.
Storage conditions
The drug Arthrotek should be stored in a place away from moisture and direct sunlight. Out of reach for children, in original packaging and at temperatures up to +25°C.
Best before date
Shelf life: 36 months.
Vacation category
Dispensed without a prescription.
Arthrotek in the treatment of psoriatic arthritis
The drug arthrotek, which is an enteric tablet containing 50 mg of diclofenac sodium and 200 mg of a synthetic analogue of prostaglandin E-1-misoprostol, maintains prostaglandin balance in the stomach and duodenum and thereby prevents the inhibitory effect of diclofenac on the protective barrier functions of the gastric mucosa
Despite the huge number of works devoted to the study of the pathogenesis and therapy of psoriasis, this problem remains relevant to this day.
This is explained by the significant prevalence of this process in the world and the increase in its severe forms, including those with joint damage - up to 61% of the total number of patients [2, 3, 6].
In the complex therapy of psoriatic arthritis, non-steroidal anti-inflammatory analgesics (NSAIDs) are often used - diclofenac sodium, brufen, naproxen, piroxicam, etc. However, the side effects from the gastrointestinal tract observed when taking them (pain, gastropathy, bleeding) often limit the possibility use of these drugs.
It has been established that the main cause of side effects is the suppression of the protective barriers of the gastric mucosa (inhibition of cyclooxinase-1 and, as a consequence, a decrease in the synthesis of endogenous prostaglandin E-1 (PGE-1) in the stomach). As is known, PGE-1 ensures the production of protective mucus rich in bicarbonates in the stomach, improves gastric blood flow, and reduces the production of hydrochloric acid. The danger of side effects that develop as a result of long-term use of NSAIDs is the asymptomatic development of lesions of the mucous membranes of the stomach and duodenum (petechiae, erosions, ulcers), ending in bleeding. Such manifestations are called NSAID-induced ulcers and gastropathy [1].
In this regard, our attention was drawn to the drug arthrotek, which is an enteric tablet containing 50 mg of diclofenac sodium and 200 mg of a synthetic analogue of prostaglandin E-1 - misoprostol. The latter, due to its rapid solubility, maintains the prostaglandin balance in the stomach and duodenum and thereby prevents the inhibitory effect of diclofenac on the protective barrier functions of the gastric mucosa [4, 5]. We used arthrotek to treat patients with psoriatic arthritis and compared its effect with the previously used drug ortofen (diclofenac sodium without misoprostol).
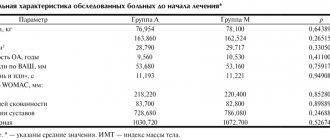
60 patients with psoriatic arthritis were observed. In all cases, the diagnosis was confirmed both clinically and radiologically.
Among the examined patients there were 29 men and 31 women aged from 16 to 66 years.
In most cases, the symptoms of psoriatic arthritis were combined with the progressive course of the psoriatic process on the skin. The duration of joint disease ranged from 1 year to 15 years or more.
Clinically, psoriatic arthritis was characterized by stiffness (39 patients), increased volume of joints (37 patients), their deformation (6 patients), severe pain (38 patients), and limitation of movement in the affected joints (38 patients).
Moreover, only large joints were affected in 21 patients, small joints in 14 patients, large and small joints in 25 patients. It should be noted that the interphalangeal joints of the hands and feet were predominantly involved in the process. 34 patients had symmetrical joint damage.
From the laboratory parameters in the general blood test, we observed leukocytosis up to 10-20x109 l., an increase in ESR (more than 65 mm/h). Biochemical parameters were characterized by a decrease in the activity of neutrophil acid phosphatase (average 143.27±12.3), neutrophil alkaline phosphatase (average 89.15±7.8), lymphocyte acid phosphatase (average 159.83±9.7). Along with this, a decrease in albumin levels and an increase in triglyceride activity were noted.
Radiologically, among the most common manifestations that we found were narrowing of joint spaces, periarticular osteoporosis of varying severity, and osteophytes.
Arthrotec was prescribed to all patients one tablet (50 mg diclofenac and 200 mg misoprostol) three times a day with meals for 30 days. Our clinical observations showed that the positive effect of the drug was observed from 12 to 15 days after the start of treatment. By the 15th day of treatment, joint pain completely stopped in two patients, and in another 12 they sharply decreased. In four patients, swelling of the joints and morning stiffness disappeared and the range of movements sharply increased. As treatment continued, the number of patients with pronounced clinical signs of improvement increased.
| Artrotek is a nonsteroidal anti-inflammatory drug that is an effective agent in the complex treatment of proriatic arthritis. |
After a 30-day course, the need to continue treatment disappeared in 16 patients, a marked improvement was observed in 17 patients; In 14 patients, joint pain stopped, and in 13 it significantly weakened. At the same time, swelling of the joints, limitation of movements, as well as morning stiffness disappeared in all patients.
All patients tolerated the treatment well, without any side effects or complications.
The normalization of clinical and biochemical parameters of blood and urine observed during treatment coincided with changes in the clinical status of the patients.
Thus, the studies conducted allow us to conclude that the non-steroidal anti-inflammatory drug arthrotek can become an effective agent in the complex treatment of psoriatic arthritis.
Literature 1. Alekseev V. G., Yakovlev V. A., Polunina T. E. Prevention and treatment of ulcerations in the stomach and duodenum caused by non-steroidal anti-inflammatory drugs // Doctor. 1996. No. 10. P. 34-37. 2. Badokin V.V. Clinic and diagnosis of psoriatic arthritis // Therapeutic. archive. M., 1977. No. 1. P. 14-19. 3. Bobyleva S.I. Clinical and pathogenetic substantiation of psoriatic arthritis and improvement of the method of its therapy: Abstract of thesis. dis. M., 1989. 4. Nasonova V. A. Arthrotek – experience of application in Russia // Medical market. M. No. 4. pp. 46–47. 5. Polunina T. E., Rakov A. A., Barsukov S. F. Safety and effectiveness of arthroteca in rheumatoid arthritis and osteoporosis // Klin. pharm. and therapist M., 1998. No. 2. P. 92–94. 6. Hilal E. A. Features of psoriatic arthritis as a manifestation of the systemic nature of the disease: Abstract of thesis. Ph.D. dis., 1998.
Arthrotec
Diclofenac is displaced by acetylsalicylic acid from the sites of its binding to albumin, which leads to a decrease in the concentration of diclofenac in plasma, as well as a decrease in its maximum concentration in plasma and a decrease in the AUC value. In this regard, the simultaneous use of diclofenac/misoprostol and acetylsalicylic acid is not recommended.
Elevated digoxin levels were reported in patients receiving concomitant digoxin and diclofenac. Therefore, patients receiving concomitant digoxin and diclofenac/misoprostol should be monitored medically for digoxin toxicity.
NSAIDs may weaken the natriuretic effect of diuretics. Since an increase in serum potassium levels may be observed when used concomitantly with potassium-sparing diuretics, in such cases it is necessary to monitor the level of this indicator. Caution must be exercised when prescribing diclofenac/misoprostol simultaneously with the above drugs.
A number of NSAIDs have been found to interact with oral anticoagulants, although it has been noted that diclofenac does not interact with anticoagulants such as warfarin. When taking indirect anticoagulants (warfarin) with NSAIDs, the risk of bleeding from the gastrointestinal tract increases. Therefore, patients receiving this type of anticoagulant concomitantly with diclofenac/misoprostol should be monitored for anticoagulant dosage adjustments if necessary.
NSAIDs may reduce the effect of antihypertensive agents, including ACE inhibitors. When diclofenac/misoprostol is co-administered with ACE inhibitors, renal function may deteriorate.
Diclofenac does not interfere with glucose metabolism in healthy people; in addition, with simultaneous use of diclofenac with hypoglycemic agents for oral administration, the effect of the latter does not change. However, there are reports of changes in the effect of such drugs when taken simultaneously with NSAIDs. Therefore, Artrotek should be prescribed with caution to patients receiving insulin or oral hypoglycemic agents.
Caution should be exercised when prescribing methotrexate concomitantly with NSAIDs, including Arthrotec, since NSAIDs may increase plasma levels of methotrexate.
Diclofenac reduces the renal clearance of lithium and increases the concentration of lithium in plasma. Therefore, Arthrotec should be prescribed with caution to patients receiving lithium.
Antacids may slow down the absorption of diclofenac. Magnesium-containing antacids may exacerbate misoprostol-associated diarrhea.
Cyclooxygenase inhibitors, such as diclofenac, due to their effect on renal prostaglandins, may increase the nephrotoxicity of cyclosporine.