Zometa and Bonviva (bisphosphonates) are often prescribed for the treatment of osteoporosis and the fight against metastases in oncology. Like all medications, they have some side effects that patients who have dental problems or are planning dental treatment in the future should be aware of. The chief physician of Dial-Dent, Sergei Vladimirovich Tsukor, gives several recommendations to such patients. In this article, he will talk about the tactics of behavior of patients planning an appointment, taking or taking the drugs Zometa and Bonviva.
What are bisphosphonates?
Bisphosphonates are a large group of drugs that stop bone loss. This group includes many different drugs with different names from different manufacturers, including Zometa and Bonviva.
In a healthy bone, from birth to death, two processes occur in parallel: the construction of new bone by osteoblasts (special bone tissue-building cells) and the thinning of bone by osteoclasts (bone killer cells). These two processes are related to each other like day and night. The processes of building new bone and thinning old bone must be in complete balance. Any imbalance, dominance or shutdown of one of the processes indicates illness. In a healthy person, due to the renewal of bone tissue, the entire skeleton is replaced in six months! Bone is a tissue that regenerates 100% precisely due to metabolic processes.
Bisphosphonates interfere with the balance of normal processes of bone renewal (thinning of old and building new). Bisphosphonates kill bone-destroying cells (osteoclasts). The bone loss process stops. But along with it, the ability of the bone to regenerate is also turned off! The bone seems to be there, and it seems to be stronger, because osteoclasts do not act. But in any situation where the restoration potential of the bone is needed, for example, a fracture or tooth extraction, bone restoration does not occur, since bisphosphonates “turned off” the renewal. A number of very unpleasant complications can occur, including suppuration of the bone - osteomyelitis.
You need to understand that bisphosphonates are a great discovery of medicine that have saved many people’s lives! Bisphosphonates are very effective in killing the growth of bone metastases, for example, in breast cancer. Therefore, for oncological diseases that cannot be treated surgically, taking bisphosphonates is justified. But the problem is that bisphosphonates are not always prescribed in extreme cases! Taking bisphosphonates for osteoporosis seems questionable, since in this case they do more harm than good. With osteoporosis, there is no question of life and death; there are many ways to prevent this condition. And taking bisphosphonates will make any surgical dental treatment affecting the bone impossible in the future, since the bone simply will not heal!
Even a single dose of bisphosphonates is a lifelong contraindication to dental implantation! There may be problems with bone healing even after banal tooth extractions. If, nevertheless, the benefits of taking bisphosphonates are more important than the harm, then you need to carefully prepare for their use.
Bonviva®
Osteoporosis can be confirmed by identifying low BMD (T index < -2 SD [Standard deviation]), fracture (including a history) or low bone mineral density (T index < -2.5 SD) in the absence confirmed fracture.
Hypocalcemia
Before using Bonviva®, hypocalcemia and other disorders of bone metabolism and electrolyte balance should be corrected. Patients should consume sufficient amounts of calcium and vitamin D. If the patient does not receive enough calcium and vitamin D from food, then additional calcium and vitamin D should be taken in the form of dietary supplements.
Anaphylactic reactions
Cases of anaphylactic reactions/shock, including death, have been reported in patients treated with intravenous ibandronic acid.
During intravenous administration of Bonviva®, the patient's condition should be monitored and appropriate medical care should be available. If an anaphylactic or other severe hypersensitivity/allergic reaction is detected, the infusion should be interrupted immediately and appropriate treatment should be initiated.
Patients with heart failure
Overhydration should be avoided in patients at risk of developing heart failure.
Patients with kidney failure
Serum creatinine should be determined before each injection. Patients with underlying medical conditions receiving nephrotoxic therapy who may experience deterioration in renal function should be closely monitored.
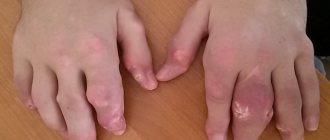
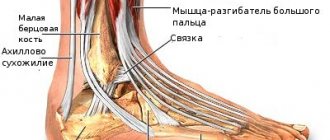
Osteonecrosis of the jaw
When bisphosphonates were used in patients with cancer, osteonecrosis of the jaw was observed, most often associated with tooth extraction and/or local infection (in particular, osteomyelitis). Osteonecrosis of the jaw developed mainly due to intravenous use of bisphosphonates, which was often accompanied by chemotherapy and the use of glucocorticosteroids.
Osteonecrosis of the jaw has also been reported with oral bisphosphonates for the treatment of osteoporosis.
In the presence of associated risk factors such as cancer, radiation or chemotherapy (including angiogenesis inhibitors), use of glucocorticosteroids, and poor oral hygiene, a dental examination and appropriate preventive treatment are recommended before prescribing bisphosphonates.
During treatment with bisphosphonates, invasive dental procedures should be avoided.
Dental surgery during bisphosphonate therapy may increase the manifestation of osteonecrosis of the jaw. It is not known whether discontinuation of bisphosphonates reduces the risk of osteonecrosis. The decision to conduct treatment must be made for each patient individually after assessing the risk/benefit ratio.
Cases of osteonecrosis of other maxillofacial areas, including the external auditory canal, have been reported in patients receiving bisphosphonate therapy, including ibandronic acid. Additional risk factors may include repeated minor injuries (eg, regular use of Q-tips). Risk factors for the development of osteonecrosis coincided with those for osteonecrosis of the jaw. Patients receiving bisphosphonates who have hearing impairment, including chronic ear infections, should be monitored for the development of osteonecrosis.
Atypical hip fractures
Atypical subtrochanteric and diaphyseal femoral fractures have been reported in patients receiving bisphosphonates, primarily in patients receiving long-term treatment for osteoporosis.
Transverse and short oblique fractures can be localized along the entire length of the femur from the lesser trochanter to the supracondylar eminence. Atypical fractures occur spontaneously or as a result of minor injuries. In the weeks or months before a complete hip fracture occurs, some patients experience hip or groin pain, which is often accompanied by radiographic evidence of a stress fracture. Because atypical fractures are often bilateral, it is necessary to monitor the other hip in patients with a femoral shaft fracture. Poor healing of atypical fractures was noted.
If an atypical fracture is suspected and pending examination results, discontinuation of bisphosphonate therapy should be considered based on an assessment of the benefit/risk ratio in each individual case. Patients should be advised to report any hip or groin pain during bisphosphonate therapy. If these symptoms are present, it is necessary to conduct an examination to identify an incomplete hip fracture.
When taking bisphosphonates, including Bonviva®, severe pain syndrome may occur: pain in the joints, bones and muscles. Pain occurred both within 24 hours and several months after starting the drug; in most patients it resolved after stopping therapy; in some of them, symptoms recurred after re-administration of the same or a different drug.
Destruction Instructions
Destruction of syringes/needles
When using and disposing of syringes and other medical products containing needles, the following rules should be strictly observed:
- do not reuse syringes and needles;
— all used needles and syringes should be placed in containers (disposable, puncture-resistant containers);
— it is necessary to store the container in places inaccessible to children;
— Disposal of needle containers with household waste should be avoided;
- Containers filled with syringes/needles should be disposed of according to local regulations or as directed by a physician.
Patients should be provided with puncture-resistant containers for disposal of syringes and needles at home.
Destruction of unused medicinal product or after expiration date
The release of medicinal products into the environment should be minimized. Bonviva® should not be disposed of with wastewater or household waste. Where possible, special systems should be used to dispose of medications.
Recommendations for patients taking Zometa or Bonviva:
- If the patient comes to the dentist with a history of taking bisphosphonates, then it is necessary to notify the doctor about the fact of taking them (drugs of the group: Zometa, Bonviva, Zoledronate, Bondronat, Boniva, etc. ). There is NO statute of limitations on these medications. Even if you only took bisphosphonates once five years ago, you should tell your dentist.
- The dentist you notify about taking bisphosphonates should try to avoid any surgical dental treatment that requires bone healing (tooth extraction, dental implants, etc.). If treatment is still necessary, for example, tooth extraction, then you should be prepared for complications in order to cope with them more easily.
For all questions regarding dental treatment while taking bisphosphonates, please contact the relevant government medical institutions.
Pharmacological properties of the drug Bonviva
Pharmacodynamics. Ibandronic acid (3-(N-methyl-3-(methylpentylamino)-1-hydroxypropane)-1, 1-diphosphonic acid monosodium monohydrate) is a highly active nitrogen-containing bisphosphonate, an inhibitor of bone resorption and osteoclast activity. Ibandronic acid prevents the development of bone destruction caused by blockade of gonadal function, retinoids, tumors and tumor extracts in vivo . Does not interfere with bone mineralization when administered in doses 5000 times higher than those used for treatment of osteoporosis. Does not affect the process of replenishment of the osteoclast pool. The selective effect of ibandronic acid on bone tissue is due to its high affinity for hydroxyapatite, which constitutes the mineral matrix of bone. Ibandronic acid inhibits bone resorption in a dose-dependent manner and does not have a direct effect on bone formation. In women during menopause, it reduces the increased rate of bone tissue renewal to the level of reproductive age, which leads to a progressive increase in bone mass, a decrease in the breakdown of bone collagen in urine and blood serum, the incidence of fractures and an increase in bone mineral density. The high efficacy and wide therapeutic range of ibandronic acid allow the use of flexible dosing regimens and periodic treatment regimens with long intervals without taking the drug in relatively low doses. The results of histological analysis of samples obtained from bone biopsies after 2 and 3 years of treatment in postmenopausal women indicate a normal state of bone tissue. In addition, there was no evidence of mineralization deficiency. Pharmacokinetics Absorption. After oral administration, ibandronic acid is rapidly absorbed from the upper gastrointestinal tract. Plasma concentrations increase proportionally to the dose up to 50 mg when administered orally. Maximum concentrations in blood plasma are achieved after 30 minutes - 2 hours (on average after 1 hour) when taken on an empty stomach, absolute bioavailability is about 0.6%. Absorption is impaired when taken simultaneously with food or drinks (except plain water). Bioavailability is reduced by approximately 90% when consuming a normal breakfast compared to bioavailability when taking the drug on an empty stomach. If ibandronic acid was taken 60 minutes before meals, no significant decrease in bioavailability was noted. When eating or drinking less than 60 minutes after taking the drug, the increase in both bioavailability and bone mineral density is reduced. Distribution. After initial systemic distribution, ibandronic acid is rapidly bound to bone tissue or excreted in the urine. 40–50% of the amount of the drug circulating in the blood penetrates well into bone tissue and accumulates in it. About 85% of the drug binds to blood plasma proteins. Metabolism. There are no data on the metabolism of ibandronic acid in animals and humans. Excretion. Ibandronic acid is eliminated from the bloodstream by bone absorption (40–50%), the rest is excreted unchanged through the kidneys. That part of ibandronic acid that is not absorbed is excreted unchanged in the feces. On average, the half-life ranges from 10 to 72 hours. The initial level of the drug in the blood plasma decreases rapidly and reaches 10% of the maximum value within 8 hours after oral administration. The total clearance of ibandronic acid is 84–160 ml/min. Renal clearance (about 60 ml/min in healthy postmenopausal women) accounts for 50–60% of the total and depends on creatinine clearance. The difference between total and renal clearance reflects the absorption of the drug into bone tissue. Pharmacokinetics in special cases Gender. The bioavailability and pharmacokinetics of ibandronic acid do not depend on gender. Race. There are no data on clinically significant interethnic differences between Mongoloid and Caucasian patients regarding the distribution of ibandronic acid. There is insufficient data on black patients. Patients with renal failure. The renal clearance of ibandronic acid in patients with various stages of renal failure depends linearly on creatinine clearance. In patients with moderately severe renal impairment (creatinine clearance ≥30 ml/min), the dose of the drug does not need to be adjusted. In persons with severe renal impairment (creatinine clearance ≤30 ml/min) who received ibandronic acid orally at a dose of 10 mg/day for 21 days, an increase in the concentration of the drug in the blood plasma was noted by 2–3 times compared with patients with unchanged renal function (creatinine clearance - 129 ml/min). The total clearance of ibandronic acid was reduced to 44 ml/min in subjects with severe renal impairment. Patients with liver failure. There are no data on the pharmacokinetics of ibandronic acid in individuals with impaired liver function. The liver does not take a significant part in the clearance of ibandronic acid, which is not metabolized, but is excreted through the kidneys and by absorption into bone tissue. Thus, in patients with impaired liver function, no dose adjustment of the drug is required. Since plasma protein binding of ibandronic acid at therapeutic concentrations is low (85%), it is unlikely that hypoproteinemia in severe liver disease will lead to a clinically significant increase in free drug concentrations. Elderly patients. The studied pharmacokinetic parameters do not depend on age. Because kidney function declines with age, this is the only factor that should be taken into account (see Patients with Renal Failure). Children. There are no data on the use of Bonviva in patients under 18 years of age.
Bonviva drug interactions
Interactions with food Food products containing calcium and other polyvalent cations (aluminum, magnesium, iron), including milk and food additives, may interfere with the absorption of the drug, so they can be consumed no earlier than 60 minutes after taking Bonviva. Interaction with other drugs Calcium preparations, antacids and some other oral drugs that contain polyvalent cations (aluminum, magnesium, iron) may interfere with the absorption of Bonviva. Therefore, the interval between taking Bonviva and other oral medications should be at least 60 minutes. Pharmacokinetic interaction studies in postmenopausal patients demonstrated the absence of any interaction with tamoxifen or hormone replacement therapy (estrogens). No interaction was observed when taken concomitantly with melphalan/prednisolone in patients with multiple myeloma. Ranitidine, when administered intravenously, increases the bioavailability of ibandronic acid by approximately 20%. No dose adjustment of Bonviva is required when taken concomitantly with H2 receptor blockers or other drugs that reduce gastric acidity. Ibandronic acid does not affect hepatic P450 isoenzymes. Binding to plasma proteins when taking the drug is insignificant. Ibandronic acid is eliminated by renal excretion and does not undergo biotransformation. The elimination pathway of ibandronic acid does not include any transport systems that are involved in the elimination of other drugs.