Intra-articular injections of Fermatron
is an effective and safe method of treating many different diseases of the musculoskeletal system by introducing a medicinal product based on hyaluronic acid into the affected joint. Fermatron injections prevent the development of degenerative processes in cartilage and joint tissues, relieve pain, and also help restore joint mobility. Health Workshop specialists actively use Fermatron injections for the treatment of arthrosis, gonarthrosis of varying severity and other joint diseases.
Fermatron, 1 piece, 2 ml, 1%, synovial fluid prosthesis
Intra-articular.
Injections should only be performed by a physician trained in the appropriate technique.
Before administration, the injection site must be treated with an antiseptic.
If joint effusion is present, it should be aspirated prior to injection.
The contents of the syringe are sterile. The insertion should be made using a sterile needle of the appropriate caliber (it is recommended to use from 18 to 21G/19 to 20G) (for Fermatron® Plus synovial fluid prosthesis, Fermatron® C synovial fluid prosthesis/Fermatron® synovial fluid prosthesis, respectively).
The syringe has a Luer Lok™
(6%) to securely attach the needle to the syringe.
Dispose of the syringe and needle after a single use.
Fermatron® Plus synovial fluid prosthesis
Fermatron® synovial fluid prosthesis
For patients with mild to moderate osteoarthritis of the knee joints, it is recommended to inject 2 ml of the drug into the synovial cavity of the knee joint once a week for 1–3 weeks for Fermatron® Plus synovial fluid prosthesis, 1–5 weeks for Fermatron® synovial fluid prosthesis.
The healthcare professional should clarify the dosing regimen for injections into the synovial space of the hip, ankle and shoulder joints.
It is recommended to perform injections into the hip, ankle and shoulder joints under ultrasound or fluoroscopic guidance.
The duration of effect in patients with mild or moderate knee osteoarthritis is up to 6 months.
The duration of the effect when administered into the hip, ankle and shoulder joints has not been established.
Fermatron® C synovial fluid prosthesis
The administration regimen is a single injection of 3 ml Fermatron® C synovial fluid prosthesis, contained in 1 syringe, into the affected synovial space.
Injected only into the joint cavity.
When injecting into the hip joint, precise placement and insertion of the needle should be ensured using fluoroscopic or ultrasound guidance.
When inserting a needle, especially for injections into the hip joint, local anesthesia is recommended.
The same needle should be used for aspiration of joint effusion and intra-articular injection.
The duration of effect in patients with mild or moderate knee osteoarthritis is up to 6 months.
What is a fermatron?
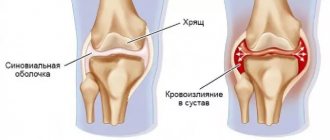
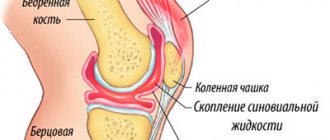
Fermatron is an analogue of human synovial fluid. With degenerative-dystrophic diseases in the joint, metabolism is disrupted, the concentration of hyaluronic acid decreases, and the articular cartilage begins to dry out and crack. As a result, a person experiences pain and difficulty moving, and joint mobility noticeably decreases. Fermatron injections restore nutrition to cartilage and reduce friction of articular surfaces against each other, improve the quality and quantity of synovial fluid.
Injections of a drug based on hyaluronic acid are made into the joint cavity using a thin needle. Once in the joint, the fermatron acts as a lubricant, thereby relieving pain and significantly improving its mobility and shock-absorbing properties. In its composition, fermatron corresponds to natural sodium hyaluronate, and accordingly has high compatibility with body tissues. All this makes this drug quite effective and makes it an excellent alternative to surgery.
Forms of release of the drug
Available in the form of a solution for a one-time injection into the joint. Contained in a disposable sterile glass syringe . Its needle is protected with a rubber cap. The drug exists in three modifications:
- Fermathron No. 1 (2 ml, syringe – 1%).
- Fermatron Plus (2 ml, syringe – 1.5%).
- Fermathron S (3 ml, syringe – 2.3%).
The difference is the capacity of the syringe that contains the medicine. All modifications are stored under general conditions. The drug contains:
- One active substance: sodium hyaluronate (2 or 3 ml depending on modification).
- Sodium chloride.
- Phosphates.
- As an excipient: water for injection.
The injection can also be given at home; there is no special need for hospitalization to use the drug. In addition, injections can be done on an outpatient basis, visiting a clinic and a doctor who can administer such an injection. During the entire treatment, the patient can carry out daily activities.
Indications for the procedure:
- arthrosis 1 and 2 degrees;
- osteoarthritis;
- after arthroscopic interventions;
- stiffness of the knee or other synovial joint
- prevention of traumatic and degenerative changes in joints.
In the Health Workshop, fermatron injections into the joint are performed by an orthopedic traumatologist. At the first session, he determines the degree of joint deformation, as well as the number of injections required to achieve a lasting positive result. Typically, the course of treatment varies from 1 to 5 injections. After the first procedure, the patient may experience swelling at the injection site or pain in the periarticular tissues.
In general, the procedure is easily tolerated and has minor contraindications.
Five years of experience in using the drug "Fermatron" in the treatment of gonarthrosis
Medical and health. Moscow Lizanets Yu.M., Bogomazov A.M., Sukhorukov E.A.
From 2003 to 2008 at the Yuzhny MOC, local therapy was carried out for 148 patients suffering from stages I-III of gonarthrosis, confirmed by Rg-logical or ultrasound data, (w/s) hyaluronic acid preparations - “synovial fluid prostheses” (Fermatron and Ostenil). Of these, 97 (65.5%) were women and 51 (34.5%) were men. During the initial examination, 86% of patients observed local inflammatory changes in the form of local hyperthermia, pain with passive movements, and signs of limited synovitis. Treatment of these patients, to relieve inflammation before local therapy, began according to the following scheme:
1) To relieve pain and relieve signs of inflammation, NSAIDs (Movalis Nimesil) were used, for topical use “Dicloran +”, Dolgit, Bystrum gel.
2) Chondroprotectors (Alflutop, Tsel T, Teraflex, Dona, Chondroitin sulfate, Artra according to the regimen recommended by the manufacturer). Local ointment forms (Chondroitin, Chondraxid).
3) If secondary synovitis is detected, arthrocentesis is performed. In the absence of signs of infection of the process, local therapy is used: a single intravenous administration of glucocorticosteroids (Diprospan solution).
4) In order to improve microcirculation, pentoxifylline preparations (Trental, Vazonit, Agapurin) were used
5) In case of severe pain, it is recommended to use a cane to relieve the sore joint.
6) Recommendations for normalizing body weight (diet, acupuncture).
7) Exercise therapy, massage, physiotherapy (Laser therapy on the Milta device, magnetic therapy).
After the elimination of signs of inflammation in the affected joint, patients were injected with hyaluronic acid preparations at stages II and up to 4 injections, at stage III 5 injections of the drug with an interval of 1 week with fixation of the joint with an orthosis. . Patients who were administered Fermatron were assigned to the first group, their number was 71 people, and the second group included 77 people. with which Ostenil was used. The comparative table took into account such parameters as a decrease in pain, an increase in volume in the affected joint and the duration of the positive effect. Within 12 months, the results of the treatment were monitored in 102 patients (≈≈69%).
| Signs of effectiveness of the use of drugs: | I – group (Fermatron) 71 patients | II-group (Ostenil) 77 patients | ||||
| 1 tbsp. | II Art. | III class | 1 tbsp. | II Art. | III Art. | |
| Pain syndrome | — eliminated in 85% of patients after 3 injections (at stage I) and after the 5th in 56% (at stage) | — eliminated in 75% of patients after 3 injections (at stages I-II) and after the 5th in 56% (at stage III) | ||||
| Restoring joint function | -I-IIst. 92%, IIIst-42% | — I-II stage - 80%, III stage - 34% | ||||
| Progression of the disease: - persistent therapeutic effect - up to 6 months. - up to 12 months | - I-IIst. 60%, IIIst-42% - I-IIst. 40%, IIIst-25%. | - I-IIst. 51%, IIIst-29% - I-IIst. 30%, IIIst-15% | ||||
Conclusions: 1) In the complex of symptomatic therapy of slowly progressing diseases of the knee joints of a dystrophic-inflammatory nature (gonarthrosis), intra-articular administration of hyaluronic acid preparations provides a good therapeutic effect. The most effective of which was "Fermatron" by 14% compared to the drug "Ostenil".
Contraindications to the procedure:
- Pregnancy and breastfeeding period;
- Hypersensitivity to the components of the drug;
- Skin diseases, the presence of abrasions and wounds in the affected area;
- Inflammatory process in the joint;
- Childhood.
Intra-articular injections of Fermatron Treatment with injections of Fermatron into the joint brings more benefits when combined with other procedures. For a speedy recovery of the patient, doctors at the Health Workshop clinics in St. Petersburg prescribe injections along with other methods:
Shelf life of Fermatron
An injection of solution for a single intra-articular injection, in a sealed package, retains its properties for no more than 48 months . Opened ampoule: within a few minutes after opening the injection capsule, the injection should be given no longer than 3-5 minutes later . It is forbidden to divide the capacity of one syringe into several doses and use it after subsequent storage.
Date location on the package : on the side with a barcode printed on the cardboard box, and on the sealed packaging of the injection syringe - on the back of the blister. Additionally, on the seal that seals the cardboard packaging.
The effect of temperature changes on storage duration: cooling below +2°C and heating above +25°C spoil the drug. It becomes unusable after any temperature change outside the permissible range.
The use of injections several days before the expiration date must be discontinued, since it may not only lose its medicinal properties by then, but also harm the patient.
Signs of unsuitability of the drug Fermatron:
- Damaged cardboard or plastic packaging with a syringe;
- A torn or re-glued paper seal with the batch number and release time data;
- The expiration date on the plastic blister does not correspond to the dates printed on the seal or cardboard packaging;
- Any inconsistencies that are in the instructions or on other information elements of the drug, errors, etc.
If the injections are expired , then they become harmful and must be disposed of. The use of such a drug threatens the deterioration of the patient’s condition, the development of inflammation and disorders in the knee joint.
How to store the drug
- The instructions for the medicine say that it must be stored in a dark place where light does not penetrate.
- The storage temperature is quite flexible ( from 2 to 25 °C ).
- It can be stored in the refrigerator, but the drug should not be frozen, otherwise it will spoil.
- If the temperature drops below 2 and rises above 25 ° C, then the medicine deteriorates and is no longer suitable for use.
- It is forbidden to hide the substance in the freezer.
- It is also best to avoid storing in an area near a stove or other heat source.
- The humidity of the storage area should be no more than 75% . Otherwise, the cardboard packaging in which the drug is located will lose its appearance.
- From the date of release, the medicine (provided that it is sealed) can be stored for up to 48 months .
- The shelf life of the opened product is several minutes . That is, the injection should be given no more than 5 minutes after the substance was opened.
- It is strictly forbidden to divide one dose of the drug into several and try to use it in the future.
- The injection should not be used several days before the expiration date. At best, the drug will simply lose all its therapeutic functions, and at worst, it will harm the person , worsening his condition and destroying the joint.
- The syringe, placed in a plastic blister, is disposable and cannot be reused. Otherwise, sterility will be compromised.
- The syringe cannot be re-sterilized.
- It is best to hide the substance from children and pets in an inaccessible place.
- You can also store the medicine in the closet. There is not much difference between it and the refrigerator.