Pharmacological properties of the drug Artron triactiv forte
Stimulates the regeneration of cartilage tissue. Glucosamine and chondroitin take part in the biosynthesis of connective tissue, preventing the processes of cartilage destruction and stimulating tissue regeneration. Glucosamine enhances the formation of the cartilage matrix and provides nonspecific protection against chemical damage to cartilage. Glucosamine is part of the endogenous glycosaminoglycans of cartilage tissue. Also, the function of glucosamine is to protect the affected cartilage from metabolic destruction caused by the use of NSAIDs and corticosteroids, in addition, it also has a moderate anti-inflammatory effect. Chondroitin sulfate sodium, regardless of whether it is absorbed in intact form or in the form of separate components, serves as an additional substrate for the formation of a healthy cartilage matrix. Stimulates the formation of hyaluron, the synthesis of proteoglycans and type II collagen, and also protects hyaluron from enzymatic breakdown (by inhibiting the activity of hyaluronidase) and from the damaging effects of free radicals; maintains the viscosity of synovial fluid, stimulates cartilage repair mechanisms and inhibits the activity of those enzymes (elastase, hyaluronidase) that break down cartilage. In osteoarthritis, it reduces the severity of symptoms of the disease and reduces the need for NSAIDs. Methylsulfonylmethane is a substance containing sulfur. Sulfur promotes the absorption of minerals by the liver, is important for brain function, the formation of hair, nails, connective tissue, cartilage, and has a pronounced anti-allergic effect. Methylsulfonylmethane promotes the removal of lactate from tissues and toxins, which improves the permeability of nutrients; Helps the body restore damaged skin cells and connective tissue, increases their elasticity. The bioavailability of glucosamine when administered orally is 25% (first pass effect through the liver). Distributed in tissues: maximum concentration in the liver, kidneys and articular cartilage. About 30% of the dose taken is distributed over a long period of time in bone and muscle tissue. Excreted primarily in urine unchanged; partially - with feces. The half-life is 68 hours. When chondroitin sulfate sodium is administered orally simultaneously in a dose of 0.3 g, its concentration in the blood plasma increases sharply. Absolute bioavailability - 12%. About 10 and 20% of the dose taken is absorbed in the form of high molecular weight and low molecular weight derivatives, respectively. Volume of distribution is about 0.44 ml/g. Chondroitin sulfate sodium is metabolized by desulfuration (after administration of low molecular weight chondroitin sulfate sodium). Excreted in urine. The half-life is 310 minutes.
Buy Ripart Forte synovial fluid prosthesis 15 mg/ml 3 ml No. 1 in pharmacies
product name
“Medicine for replacing synovial fluid RIPART®, volumes 1 ml, 2 ml, 3 ml, RIPART® Forte, volumes 1 ml, 2 ml, 3 ml and RIPART® Long, volumes 1 ml, 2 ml, 3 ml” (hereinafter referred to as text - Means for replacing synovial fluid).
Type of contact with the human body: constant contact with the internal environment of the body.
This product is sterile. For single use.
Conditions of use: in clinics, hospitals and other medical institutions by qualified medical personnel trained in the technique of injecting synovial fluid replacement agents.
Application area
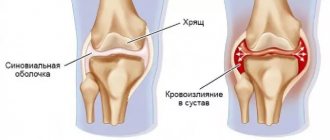
The product is used for administration to replace synovial fluid in case of joint damage, to eliminate pain and improve joint mobility. The agent for replacing synovial fluid is used for osteoarthritis and other degenerative-dystrophic, traumatic and post-traumatic changes in the joints: knee, hip and other large joints, as well as as an aid in orthopedic surgery.
The medical device is used in traumatology, orthopedics, and sports medicine.
Product characteristics
The synovial fluid replacement product is a sterile, colorless and transparent viscous solution of highly purified sodium hyaluronate, obtained by biofermentation. The product contains sodium hyaluronate in a concentration of 10 mg/ml (RIPART® synovial fluid replacement agent), 15 mg/ml (RIPART® Forte synovial fluid replacement agent) and 20 mg/ml (RIPART® Long synovial fluid replacement agent), sodium dihydrogen phosphate, disodium hydrogen phosphate, sodium chloride 0.9% solution for infusion.
Sodium hyaluronate is the sodium salt of hyaluronic acid, a glycosaminoglycan formed by groups representing D-glucuronic acid and N-acetyl-D-glucosaminodisaccharide. It is widely present in the extracellular matrix in both animals and humans. The molecular weight of sodium hyaluronate used in the manufacture of the synovial fluid replacement agent must be at least 3 MDa.
Sodium hyaluronate belongs to a small group of substances that are the same for all living organisms. Sodium hyaluronate is a natural polysaccharide that is part of all tissues of the body, and sodium hyaluronate is found in especially high concentrations in synovial fluid and skin. The synovial fluid replacement agent consists of biosynthetically produced and purified sodium hyaluronate. Sodium hyaluronate in the body is a natural component of synovial fluid, which in joints serves as a lubricant for cartilage and ligaments, and is also an shock absorber. It is known that synovial fluid in affected joints has lower viscosity and elasticity than synovial fluid in healthy joints. Injecting sodium hyaluronate into a joint to restore viscosity and elasticity can reduce pain and restore joint mobility.
The composition of the synovial fluid replacement agent ensures the restoration of viscoelastic properties and the transition of the viscous properties of the solution into elastic (elastic) in the range of shear rates from 0.5 to 2.5 Hz, which corresponds to the range of movements from walking to running.
Main technical characteristics and materials used in manufacturing
Table 1. Main technical characteristics of the product
| Index | Values | |
| 1 | Chroma | Colorless |
| 2 | Transparency | Transparent |
| 3 | Overall length (pre-filled syringe with piston), mm (± 1%) | 117 (for a syringe with a volume of 1 ml) 135 (for a 2ml syringe) 153 (for a 3ml syringe) |
| 4 | Weight of the product in individual packaging (in a syringe), g (± 10%) | 12.0 (for a syringe with a volume of 1 ml) 13.1 (for a 2ml syringe) 14.2 (for a syringe with a volume of 3 ml) |
Table 2 - Materials used in the manufacture of the product
| Product part name | Material |
| Synovial fluid replacement agent | One milliliter contains: Sodium hyaluronate 10/15/20 mg Sodium dihydrogen phosphate 0.1 mg Disodium hydrogen phosphate 0.6 mg Sodium chloride, solution for infusion 0.9% – up to a volume of 1 ml |
| Syringe: | |
| Cylinder | Borosilicate glass Type 1 |
| Cap | Elastomer BDM-PS |
| Syringe rod | Elastomer BDM-PS |
| Piston seal | Elastomer BDM-PS |
| Finger rests | Elastomer BDM-PS |
| Package | |
| Individual packing | Paper, polymer film |
| Cardboard pack | Cardboard |
Information about the presence of a medicinal product, materials of animal and (or) human origin in a medical product
The synovial fluid replacement product contains the following drugs:
- sodium hyaluronate; - sodium chloride, solution for infusion 0.9%;
Materials of animal and (or) human origin are not contained in this medical product.
Sterility
The product is supplied sterile. Sterilization parameters: steam sterilization method in accordance with the requirements of GOST R ISO 17665-1.
Repeated sterilization of the product is prohibited. Reuse is prohibited.
Indications, contraindications, warnings, precautions, side effects
Indications for use:
— Osteoarthritis and other degenerative-dystrophic, traumatic and post-traumatic changes in joints: knee, hip and other joints. — As an aid in orthopedic surgery. — For use in patients who have increased physical activity and regularly load the damaged joint.
Contraindications:
— Do not use in patients suffering from hypersensitivity (allergy) to products containing sodium hyaluronate. — It is prohibited to inject the Synovial Fluid Replacement Agent into the joint of patients who have infectious or skin diseases in the area where the injection is intended.
Warnings:
To prepare the skin, it is prohibited to simultaneously use disinfectants containing quaternary ammonium salts, since sodium hyaluronate may form a precipitate in their presence.
Intravascular administration is prohibited due to the possibility of systemic adverse side effects. It is prohibited to use the product to replace synovial fluid during intraocular surgery.
Precautionary measures:
— The safety and effectiveness of injection of this product in parallel with other intra-articular injections have not been studied. — Efficacy has been established only if the minimum recommended course of treatment is followed. — The synovial fluid replacement agent should be used with caution in patients with signs of impaired venous or lymphatic drainage in the lower extremities. — For bilateral treatment, separate syringes should be used for each knee or hip joint. — Local anesthetics should not be used if the patient is known to be allergic or hypersensitive to local anesthetics. — X-ray-guided hip injections should not be performed using radiocontrast agents if the patient is known to be allergic or hypersensitive to radiocontrast agents. — STERILE CONTENTS. The pre-filled syringe is intended for single use. The contents of the syringe must be used immediately after the container is opened. Any unused amount of Synovial Fluid Replacement Agent should be discarded. — It is necessary to check the expiration date and the integrity of the individual packaging (syringe) before using the medical product. Do not use after the expiration date, or if the packaging is open or damaged.
Side effects:
The synovial fluid replacement agent is well tolerated. Possible side effects include short-term pain at the injection site, a feeling of heat, bruising, redness and/or swelling. As a rule, such reactions disappear without a trace within 2-3 days and do not in any way affect the effectiveness of treatment. Cases of allergic and anaphylactic reactions are rare. If precautions are not taken during intra-articular administration, septic arthritis may occur in very rare cases.
Risks of use:
The manufacturer has determined the risks for the patient associated with the occurrence of each type of hazardous factors and the occurrence of their consequences. The parameters for reducing risks when applying measures to prevent the occurrence of conditions for the manifestation of hazardous factors or the occurrence of their consequences are calculated.
It has been established that after the implementation of risk control measures, no new risks arise, and the overall residual risk is considered acceptable; The production risks do not change depending on the composition and type of packaging of a medical product.
Patient Information
- As with any invasive procedures performed on the joints, the patient is advised to avoid any physical exertion, as well as prolonged (lasting for more than 1 hour) activities associated with bearing weight loads (this applies, for example, to jogging and tennis) within 48 hours after intra-articular injection of the product. — The safety and effectiveness of use of the drug for replacing synovial fluid in women during pregnancy and lactation, as well as in children under 18 years of age, has not been established.
Recommendations for a medical specialist
The volume of synovial fluid replacement agent administered depends on the size of the joint. The physician is responsible for determining the applicable volume and must ensure that the joint is not overloaded.
A course of treatment with RIPART® synovial fluid replacement agent usually requires a cycle of procedures consisting of 3-5 injections (with an interval of 1 week).
A course of treatment with RIPART® Forte, a synovial fluid replacement agent, usually requires a cycle of procedures consisting of 1-3 injections (with an interval of 1 week).
A course of treatment with RIPART® Long, a synovial fluid replacement agent, usually requires a cycle of procedures consisting of 1-2 injections (with an interval of 1 week).
The amount of sodium hyaluronate injected into the joint during the procedure is determined according to tables 3, 4 and 5. The choice of dosage is at the discretion of the attending physician.
Table 3. For RIPART® Synovial Fluid Replacement Agent
| Syringe filling volume (10 mg/ml (1%)) | The amount of sodium hyaluronate in the syringe |
| 1 ml | 10 mg |
| 2 ml | 20 mg |
| 3 ml | 30 mg |
Table 4. For Synovial Fluid Replacement Agent RIPART® Forte
| Filling volume (15 mg/ml (1.5%)) | The amount of sodium hyaluronate in the syringe |
| 1 ml | 15 mg |
| 2 ml | 30 mg |
| 3 ml | 45 mg |
Table 5. For Synovial Fluid Replacement Agent RIPART® Long
| Filling volume (20 mg/ml (2%)) | The amount of sodium hyaluronate in the syringe |
| 1 ml | 20 mg |
| 2 ml | 40 mg |
| 3 ml | 60 mg |
If necessary, the course can be repeated.
The decision on the applicability of a repeated course is made by the attending physician.
Procedure for working with a medical device
1. The synovial fluid replacement agent is administered intra-articularly once. Subcutaneous administration of lidocaine or a similar local anesthetic may be recommended prior to administration of the synovial fluid replacement agent. 2. Do not use Synovial Fluid Replacement Product if the blister pack is opened or shows signs of damage. Do not use the product after the expiration date indicated on the packaging. 3. When preparing for use and during the actual use of a medical device, strict adherence to aseptic rules is required. 4. If necessary (if present), remove joint effusion before administering Synovial Fluid Replacement Agent. 5. It is necessary to remove the screw cap of the syringe and the cap of the needle tip in compliance with all the rules of asepsis. Introduction Means for replacing synovial fluid into the joint must be carried out using an appropriately sized needle (selected based on the size of the joint). The needle is not included in the product packaging.
Inject the required volume of synovial fluid replacement into each joint. If there is a doctor's order that involves injection into several joints, a separate syringe must be used for each joint.
The synovial fluid replacement product is a DISPOSABLE MEDICAL DEVICE (REPEATED USE IS PROHIBITED).
Conditions of use, storage and transportation
The medical device is used indoors at an ambient temperature of 2°C to 25°C.
Transportation must be carried out by all types of transport, in closed vehicles, in accordance with the rules for the carriage of goods in force for this type of transport.
The synovial fluid replacement agent must be transported in the manufacturer's packaging at a temperature of 2°C to 25°C and a relative humidity of 30 to 75% without moisture condensation.
The synovial fluid replacement product must be stored in the manufacturer's packaging, protected from direct sunlight, heat and freezing, at a temperature from 2°C to 25°C and relative humidity from 30 to 75% without moisture condensation.
Biodegradation of a medical device in the patient’s body
The protective film formed after the introduction of the synovial fluid replacement agent retains its elastic properties for 6 months.
After 6 months, the synovial fluid replacement agent breaks down under the influence of a group of tissue enzymes called hyaluronidases into decomposition products: oligosaccharides and low molecular weight hyaluronates, which are subsequently excreted from the body through natural metabolic pathways.
Removal or replacement Synovial fluid replacement agents are not possible because it is inseparably mixed with the synovial fluid of the joint.
Medical product packaging
The synovial fluid replacement product is individually packaged as a syringe. Next, 1 syringe is packaged in a single-barrier polymer contour cell packaging, hermetically sealed with laminated paper or multilayer combined paper-based material. Next, single-barrier polymer contour blister packaging 1 pc. Packed in a cardboard box. Instructions for use included 1 piece.
Disposal Information
The medical device must be disposed of after use or expiration date, as well as if the integrity of the packaging is damaged, in accordance with the local legislation of the consumer’s country.
Disposal of used syringes must be carried out in accordance with SanPiN 2.1.7.2790-10 for class B waste (epidemiologically hazardous waste).
Unused medical products with an expired expiration date or with damaged packaging, as well as blister packaging, cardboard packs and shipping containers are disposed of in accordance with SanPiN 2.1.7.2790-10 for class A waste (epidemiologically safe waste, similar in composition to solid household waste) .
Guarantees
The manufacturer guarantees that the quality of the product meets the requirements of the technical specifications, subject to the conditions of use, integrity of packaging, transportation and storage conditions in accordance with the manufacturer’s instructions for use.
The warranty period when stored in packaging is 3 years from the date of production (packaging).
After the warranty period expires, the product must be disposed of.
Doctor's review
Alexander Pichugin Doctor of the highest category,
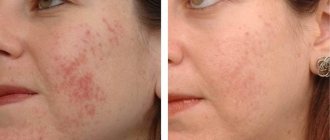
“Artron triactive is suitable for seasonal and regular use; its effect is manifested not only in eliminating allergic symptoms, but also in eliminating the causes. Even the very first procedures can relieve you from shortness of breath, cough, skin rashes and itching, runny nose symptoms, swelling, and tearfulness. Carrying out a course of therapy with this drug can eliminate allergic reactions at the peak of its exacerbation. In addition, from the use of the product you can expect the restoration of metabolic reactions, cleansing the body of poisons and toxins, increasing immune resistance, improving well-being, and increasing performance. Passed clinical studies and has certificates of conformity.”
Content
- Characteristics of artron triactive forte table, n60
- Delivery artron triactivorte
- Doctor's review of Artron triactivort
Up to 80% of people over 55 years of age complain of sore joints.
Very often, the cause of pain is premature aging of the articular cartilage, which becomes thinner and can no longer function as a shock absorber.
It is possible to slow down the aging of cartilage tissue, the main thing is to do it at an early stage.
Chondoprotectors will help you with this.
Right now you can order Artron Triactiv Forte tablets at the pharmacy.
This is a combined drug that has chondoprotective, antirheumatic, anti-inflammatory and analgesic effects.
Indications.
The active ingredients of this complex are chondroitin sulfate, methylsulfonylmethane and glucosamine hydrochloride.
Once in the body, they improve metabolism, stimulate the restoration of cartilage and bone tissue, eliminate pain and reduce inflammation.
Advice to buy Artron Triactive Forte in a pharmacy can be given to those patients who require active therapy, and to those who need prevention of the following diseases: osteochondrosis and arthrosis (damage, destruction of cartilage tissue);