People who take an active lifestyle prefer to solve the problem of osteoarthritis radically. Instead of taking chondroprotectors for many months, they choose intra-articular injections of synovial fluid substitutes. This allows you to quickly and safely return to your usual sports activities.
The problem is in choosing a drug, because today the market offers both affordable Russian liquid endoprostheses and more expensive imported ones. What to choose and why - let's look at the example of three options - "Synvisk", "RusVisk" and "Noltrexin".
Comparison of the main characteristics of "Synvisk", "RusVisk" and "Noltrexin"
| Synvisc | RusVisk | Noltrexin | |
| Number of injections per course | 3 | 3-5 | 1-4 |
| Molecular mass | 6 million Yes | 3-3.5 million Yes | 12 million Yes |
| Therapeutic effect after the course | 6-12 months | 8-10 months | Up to 24 months |
| Origin | Biofermentation method (hyaluronic acid) | Biofermentation method (hyaluronic acid) | Synthetic drug |
| Manufacturer country | USA | Russia | Switzerland |
| Efficacy in late stages | average | average and below average | high |
| Possibility of an allergic reaction | Occurs due to the presence of chicken protein (cases of anaphylactic shock have been reported) | Allergic reactions to natural components of the drug are possible | Very rare - the drug is hypoallergenic |
| The presence of silver ions in the composition has a disinfecting effect | No | No | Yes |
What is the Russian liquid endoprosthesis “RusVisk”
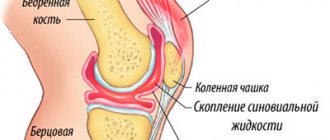
The drug appeared on the market in 2009. It is a classic liquid endoprosthesis based on sodium hyaluronate (also containing sodium chloride, sodium dihydrogen phosphate, and water). Unlike other similar medications, RusVisk has a special formula due to the stabilization of hyaluronic acid.
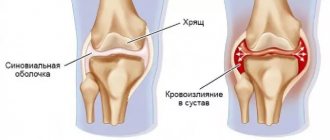
Special processing of the active substance allowed scientists to significantly increase the hydrophilic qualities of the endoprosthesis: it retains moisture well and retains it in the joint, which means it effectively solves the problem of synovial fluid deficiency in arthrosis. In addition, it has mechanical chondroprotective qualities and an antioxidant effect.
This drug has one significant drawback. Russian developers conducted a series of studies and made the following conclusions. After three injections, approximately 70% of patients experienced a significant reduction in pain during movement. While at rest the pain went away almost completely. Therefore, sports traumatologists rarely recommend this particular drug for the treatment of arthrosis of the knee, elbow or hip.
"RusVisk" is not intended for athletes: the effect is best manifested at rest
Synvisc (synovial fluid prosthesis) syringe 6ml No. 1 (GENZYME BIOSERDGERI, UNITED STATES)
Compound.
| active substance: | |
| Hylan ( Hylan GF 20) | 8 mg |
| excipients: sodium chloride - 8.5 mg; sodium hydrogen phosphate - 0.16 mg; sodium dihydrogen phosphate - 0.04 mg; water for injection - up to 1 ml |
Pharmachologic effect. Replenishes the deficiency of synovial fluid.
Effect on the body.
Synvisc I is a sterile, pyrogen-free, viscoelastic liquid containing hylans. Hylans are derivatives of hyaluronan (sodium salt of hyaluronic acid) and consist of repeating disaccharide units of N-acetylglycosamine and sodium glucuronate. The average molecular weight of hylan A is about 6 million. Yes, hylan B is a hydrated gel.
Synvisc is a biological analogue of hyaluronan. Hyaluronan is a component of synovial fluid that determines its viscoelastic properties.
Synvisc I temporarily replaces and replenishes synovial fluid; effective in patients at any stage of joint pathology; most effective in patients with active and regular load on the affected joint; achieves its therapeutic effect by restoring viscosity, thereby restoring the rheological properties of the synovial fluid and the physiological and rheological properties of the tissues of the joint affected by osteoarthritis.
Viscosity restoration is a treatment designed to reduce pain and discomfort, allowing for more extensive joint movement. In vitro studies
showed that Synvisc I protects cartilage cells from certain physical and chemical damage.
Synvisc I is intended only for intra-articular administration by a physician for the treatment of pain caused by osteoarthritis of the knee joint.
The effect of using Synvisc I applies only to the affected joint. Synvisc I has no general effect.
Data from prospective clinical studies in patients with osteoarthritis of the knee have demonstrated the effectiveness of Synvisc I for up to 52 weeks after a single injection.
Component properties.
Synvisc I contains hylan A and hylan B (8.0±2.0) mg/ml), diluted in physiological sodium chloride solution (pH 7.2±0.3). The mechanical (viscoelastic) properties of Synvisc I are higher than those of synovial fluid and hyaluronan solution in comparable concentrations.
Synvisc I has an elasticity (storage modulus G') at 2.5 Hz at (111±13) Pa and a viscosity (loss modulus G") at (25±2) Pa. The elasticity and viscosity of the synovial fluid of the knee joint in patients aged 18–27 years, measured by the same method and at the same frequency, are respectively: G'=(117±13) Pa and G"=(45±8) Pa.
Hylans are excreted from the body in the same ways as hyaluronan, and the breakdown products are not toxic.
Recommended.
Osteoarthritis of the knee joint.
Contraindications.
Synvisc I should not be injected into the knee joint if there is venous or lymphatic stasis of the limb.
Synvisc I should not be injected into an infected or severely inflamed knee joint, nor should it be used in patients with skin lesions or infection in the immediate vicinity of the injection site.
Side effects.
After an intra-articular injection of Synvisc I, the following side effects may be observed on the side of the knee into which the injection was made: short-term pain and/or swelling, and/or joint effusion.
Post-marketing experience with Synvisc has shown that in some cases joint effusion can be significant and cause more prolonged pain. It is necessary to puncture and remove exudate, followed by analysis to exclude infection and microcrystalline arthropathy. Usually the reactions go away without leaving a trace within a few days. The presence of such reactions does not affect the effectiveness of treatment.
Intra-articular infections were not observed in any of the clinical studies, and they have only rarely been reported with clinical use of Synvisc.
Post-marketing experience has revealed the following systemic reactions, which were rarely observed with the administration of Synvisc: rash, urticaria, itching, fever, nausea, headache, dizziness, chills, muscle cramps, paresthesia, peripheral edema (including facial swelling), weakness, difficulty breathing, redness of the skin.
In a controlled clinical trial, the incidence and type of side effects were found to be similar in the group of patients treated with Synvisc I and in the group treated with placebo.
Method of administration and dose.
The recommended regimen for using Synvisc I is 1 injection into the knee joint. If necessary, a second injection can be given 6 months after the first.
Synovial fluid or exudate should be removed before injecting Synvisc I.
Synvisc I should be administered at room temperature, following strict aseptic procedures, especially when removing the protective cap from the syringe.
Synvisc I should be injected strictly into the synovial space, using fluoroscopy if necessary to determine the direction of injection.
When fluoroscopy is used, ionic or nonionic contrast agent may be used to determine the direction of injection. For every 2 ml of Synvisc I, no more than 1 ml of contrast agent should be used.
The appropriate length and size (18–20) needle should be used.
Special instructions.
The drug should not be administered in the presence of significant intra-articular effusion.
As after any invasive procedure in the joint, after injection of the drug the patient is recommended to adhere to a gentle regimen, gradually restoring normal activity over several days.
The drug has not been studied in pregnant women, children and adolescents under 18 years of age.
The drug contains a small amount of chicken protein, so caution should be used when treating patients with hypersensitivity to chicken protein.
Do not use for intravascular administration.
Do not use for extra-articular injection or injection into synovial tissue or joint capsule. As a result of extra-articular administration, adverse reactions may develop in the injection area.
Do not use together with skin disinfectants containing quaternary ammonium salts, as in their presence, hyaluronan can form a sediment.
The contents of the syringe are for single use only.
Synvisc I should not be used if the packaging is opened or damaged.
When removing the syringe from the blister, it should be held by the body without touching the piston.
Before removing the cap, it should be turned to minimize leakage of the syringe contents.
To prevent leakage of the syringe contents, keep the needle in close contact with the syringe during injection.
Do not twist or apply excessive force when fixing the needle or removing the cap, as this may cause the syringe tip to break.
Repeated sterilization of Synvisc I is not permitted.
Release form.
Synovial fluid prosthesis.
6 ml in 10 ml glass syringes. 1 syringe is placed in a blister made of polymer material, hermetically sealed on top with a film. 1 bl. placed in a cardboard box.
Manufacturer.
Genzyme Biocare, a division of Genzyme Corporation. USA, 1125, Pleasant View Terrace, Ridgefield, New Jersey 07657, USA.
What to look for when choosing a drug and why
The primary indicator when choosing a liquid endoprosthesis is molecular weight. It shows the length of the molecule chain and the number of its links. The longer the molecule, the more difficult it is to break down in the joint and the longer it remains in the body. This is very indicative of our drugs:
- "RusWisk" with a molecular weight of 3-3.5 million Yes lasts no more than 8-10 months;
- “Synvisk” – 6 million Yes – gives an effect lasting up to 12 months;
- Noltrexin, which has the highest molecular weight - 12 million Da - allows you to take repeated courses no more often than once every two years.
Let’s make the minimum calculations for 2 years of treatment for hip arthrosis:
1. "RusVisk":
- It is necessary to complete 3 courses with an interval of 8 months.
- The duration of one course is 3-5 injections.
- Over 2 years, you will have to make from 9 to 15 injections , depending on the degree of arthrosis.
2. "Synvisk":
- In the best case, it is necessary to take 2 courses with an interval of 12 months (more often, patients feel the need for injections earlier, after 6-8 months).
- Duration of the course – 3 injections.
- In 2 years, you will have to do 6 injections at best, or 9 injections if arthrosis makes itself known earlier.
3. Noltrexin.
- One course is enough for 2 years.
- Duration – from 1 to 4 injections in the most severe cases.
- For 2 years, even with advanced stages of arthrosis, 4 injections , in other cases – 1-3 injections to achieve a stable result.
2 years without pain due to arthrosis - this is an absolute record, which is achieved by a course of Noltrexin injections
The fundamental difference between Noltrexin and RusWisk and Synvisk
"RusVisk" and "Synvisk" are representatives of a large group of liquid endoprostheses based on hyaluronic acid. They are produced using autoclaving technology, which accelerates the breakdown of sodium hyaluronate molecules. The composition may contain microscopic protein residues, which can cause an allergic reaction. In most cases, we are talking about redness and rash, but complications are theoretically possible, up to anaphylactic shock (such occasional cases have been recorded).
Noltrexsin is the third generation of viscoprosthesis based on a synthetic polymer. It contains no components of natural origin, so an allergic reaction is excluded. The drug includes single gel macromolecules with a record high molecular weight. The formula is unique in that the macromolecules are very tightly cross-linked. It is impossible to achieve such an effect in the case of sodium hyaluronate, therefore none of the hyaluronic acid-based preparations on the market can give such a long-lasting result - up to two years.
Injections based on hyaluronic acid are always a risk of allergies
Today, the Swiss “Noltrexin” is not yet as well known in Russia as the popular “Synvisk” and “RusVisk”. The latter is often preferred, considering it more accessible due to its Russian origin. If you are fond of sports and are looking for a liquid endoprosthesis, including for the prevention of sports injuries, which will give the longest lasting effect and will be effective even during intense physical activity, the choice in favor of Noltrexin is obvious!
Synvisc 2ml 16mg syringe No. 1 (1 piece) - Instructions
Dosage form
Prosthetic synovial fluid in glass syringes. The package contains 1 syringe.
Compound
One milliliter of Synvisc contains:
Active substance: 16 mg Hylan GF 20.
Excipients: 8.5 mg sodium chloride, 0.16 mg sodium hydrogen phosphate, 0.04 mg sodium dihydrogen phosphate, water for injection - up to 1 ml.
Pharmacological properties
Synvisc is a biological analogue of hyaluronate, a component of synovial fluid, which provides the viscoelastic characteristics of the intra-articular fluid. Synvisc I / Synvisc One (Hylan GF 20) is a sterile, pyrogen-free, viscoelastic liquid containing hylans. The mechanical properties of hylan are higher than natural synovial fluid hyaluronate and hyaluronic acid solution in similar concentrations. Gilan is excreted from the body in similar ways as hyaluronate; the breakdown products of hylan are not toxic. Synvisc replenishes the viscosity of the fluid, which improves the rheological and physiological state of the tissues of the diseased joint. Synvisc reduces pain and discomfort caused by dystrophic changes in cartilage tissue, thereby improving mobility in the joint. The ability of the drug to protect cartilage from chemical and mechanical damage has been proven.
Hylans are derivatives of hyaluronan (sodium salt of hyaluronic acid), and consist of repeating disaccharide units of N-acetylglucosamine and sodium glucuronate. Gilan A has an average molecular weight of about 6 million daltons. Gilan B is a hydrated gel. Synvisc I/ Synvisc One (Hylan GF 20) contains hylan A and hylan B (8.0 mg ± 2.0 mg per 1 ml), diluted in physiological sodium chloride solution (pH 7.2 ± 0.3).
Hylan GF 20 is a biological analogue of hyaluronan. Hyaluronan is a component of synovial fluid that determines its viscoelastic properties. However, the mechanical (viscoelastic) properties of Hylan GF 20 are superior to those of synovial fluid and hyaluronan solutions in comparable concentrations. The elasticity of Hylan G-F20 (storage modulus G') at 2.5 Hz is 111±13 Pascal (Pa), and the viscosity (loss modulus G") is 25±2 Pa.
The elasticity and viscosity of the synovial fluid of the knee joint in people aged 18-27 years, measured using a comparable method and at a frequency of 2.5 Hz, are G'=117±13 Pa and G”=45±8 Pa, respectively.
Hylans are excreted from the body in the same way as hyaluronan, and its breakdown products are not toxic.
Indications for use
Synvisc is intended for:
- temporary replenishment or replacement of synovial fluid;
- treatment of patients suffering from osteoarthritis of the knee joint (gonarthrosis) at any stage of the disease;
- effective recovery of patients who constantly load their joints and lead an active lifestyle (athletes, workers engaged in heavy physical labor);
- exclusively intra-articular injection for pain relief in osteoarthritis of the knee joint.
Directions for use and dosage
Injections of chondroprotectors should be carried out strictly in a hospital and by specialists who have a certificate for such manipulations.
During the procedure, the doctor must strictly follow all recommendations for the correct execution of the manipulation. The drug Synvisc is administered strictly into the synovial space of large joints in adults.
The syringe itself and its contents are sterile and completely ready for use. The solution should be used immediately after opening the package. Do not use the product if the container is damaged.
It is enough to give three injections for the positive effect to be noticeable. The injection is performed once a week with a break of exactly one week.
The maximum permissible dose is six injections over six months with an interval of 4 weeks.
Synvisc has a therapeutic effect only on the diseased joint where the solution is injected; no systemic effect has been detected from it.
Side effects
Immediately after administration, short-term pain and local swelling may occur, accompanied or not by intra-articular exudation. In some cases, exudation is quite abundant and causes prolonged pain. To eliminate such symptoms in case of accumulation of exudate, it must be removed from the joint cavity by puncture. Next, the contents of the articular cavity are subjected to microscopy to exclude microcrystalline arthropathy (gout) or infectious pathology.
Pain/tenderness at the injection site, bruising at the injection site, swelling at the injection site, bleeding at the injection site, itching at the injection site, redness of the skin at the injection site, rash at the injection site and localized fever at the injection site.
Hypersensitivity reactions, including anaphylactic reactions, anaphylactoid reactions, anaphylactic shock and angioedema.
Acute inflammation characterized by pain and swelling of the joint, the appearance of effusion (intra-articular exudation) and sometimes a local increase in soft tissue temperature and/or joint stiffness.
Synovial fluid analysis revealed aseptic fluid without crystals. This reaction often reverses within a few days after treatment with nonsteroidal anti-inflammatory drugs (NSAIDs), intra-articular corticosteroids, and/or arthrocentesis. Following such reactions, clinical treatment effects may still be observed.
Systemic reactions: rash, urticaria, itching, fever, nausea, headache, dizziness, chills, muscle cramps, paresthesia, peripheral edema, weakness, difficulty breathing, redness of the skin and facial swelling.
In controlled clinical studies conducted with Synvisc, there was no statistically significant difference in the number or types of systemic side effects between the group of patients receiving Synvisc and the group of patients receiving control treatments, and the incidence and types of side effects were similar between the groups patients receiving Synvisc and placebo.
Contraindications
The use of Synvisc is contraindicated in:
- venous and lymphatic stasis present in the area of the affected joint;
- the presence of an infected and inflamed joint, signs of skin infection directly in the area where the solution is supposed to be administered;
- allergic reactions and hypersensitivity to sodium hyaluronate;
- pregnancy and breastfeeding.
Interaction with other drugs
Pharmaceutical compatibility studies with injectable corticosteroids and analgesics have shown that Synvisc can be mixed with commercially available injectable corticosteroids or analgesics in a ratio of three parts Synvisc to one part injectable corticosteroid or analgesic, while maintaining its rheological properties within specifications.
Synvisc should not be mixed with other medications.
special instructions
Do not administer intravascularly (inside blood vessels).
Synvisc cannot be injected extra-articularly or into synovial tissues and the joint capsule. After extra-articular (extra-articular) injection, adverse events may occur, usually at the injection site
Do not use together with skin disinfectants containing quaternary ammonium salts, as in their presence hyaluronan may precipitate.
Synvisc should not be administered if significant intra-articular effusion is observed immediately before injection.
As after any invasive intra-articular procedure, after intra-articular injection of Synvisc I / Synvisc One, the patient is advised to avoid strenuous physical activity and resume normal physical activity for several days.
Synvisc has not been studied in pregnant women or children and adolescents under 18 years of age.
Synvisc should not be used by patients with hypersensitivity to poultry protein, as it contains a small amount of poultry (chicken) protein.
The effect of application applies only to the affected joint. Synvisc has no general effect.
Typically, the duration of effect of Synvisc in patients with knee osteoarthritis lasts for 26 weeks after one injection. However, prospective clinical data from patients with knee osteoarthritis demonstrated treatment efficacy of up to 52 weeks after a single administration of Synvisc (reduction of pain, knee stiffness and improvement of knee function).
Storage conditions
Store at temperatures from +2 °C to +30 °C. Do not freeze.
Keep out of the reach of children.
Vacation category
On prescription.