Properties
Hyaluronic acid (HA) is a physiological component of synovial fluid, is responsible for its viscosity and plays a major role in maintaining biological lubrication of the joint, and activates the processes of tissue renewal of articular cartilage.
The active substance of the medical product Suplazin is a highly purified specific fraction of unstabilized hyaluronic acid (hereinafter HA) with a certain length of the molecular chain, of bioenzymatic origin. Suplasin has viscoelastic properties similar to the synovial fluid of the joints, therefore, immediately after administration, it acts as a shock-absorbing and lubricating agent that restores painless movement of the joint.
Due to its unstabilized structure, hyaluronic acid quickly enters the extracellular matrix of cartilage, eliminating its deficiency and helping to restore the elasticity of cartilage.
The molecular chain of Suplasin GC has an optimal length to activate tissue metabolic processes. Through the interaction of hyaluronic acid with specific cell receptors (fibroblasts, chondrocytes), as a result, it quickly stimulates the synthesis of endogenous hyaluronan and has a structural-modifying effect on the course of osteoarthritis.
Doctor's review
Alexander Pichugin Doctor of the highest category,
“Suplazin can be used regularly and seasonally; it has an effect on allergic manifestations and the causes that caused them. From the first use, it eliminates lacrimation, swelling, runny nose and nasal congestion, skin itching and rashes, cough, asthmatic attacks, shortness of breath. To protect yourself from allergic manifestations during the allergy season, you need to use this drug as a course. As an additional bonus, this product has the effect of increasing performance and improving the general condition of the body, removing toxins and poisons, increasing immune capabilities and restoring metabolic reactions. This drug has participated in clinical-level studies and received the necessary certificates.”
Recommendations for use
Suplasin 1-Shot is intended to improve the physiological function of all synovial joints (knee, hip, shoulder and fingers, etc.) and symptomatic treatment of pain in:
- degenerative lesions of joint tissue (osteoarthrosis), especially for patients who are overweight or do not receive an adequate therapeutic effect from taking analgesics and anti-inflammatory drugs, physiotherapeutic procedures;
- traumatic lesions of cartilage due to sports, industrial or household trauma to the joint;
- after arthrocentesis, in order to normalize the condition of the joint.
Several joints can be treated at the same time.
Mode of application
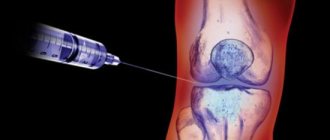
Depending on the size of the joint, up to 6 ml can be administered intra-articularly (inside the joint). Direct intra-articular administration must be carried out under strict aseptic conditions by professional medical personnel. Before use, it is necessary to warm the syringe with the solution to room temperature. Any unused parts of the solution in the syringe must not be used; they must be disposed of.
Suplasin 1-Shot: intended for one-time intra-articular administration in one course
The graduation marks on the syringe should be used as a guide only.
Content
- Characteristics of suplazin syringe 20mg/2ml
- Delivery of suplazin
- Doctor's review of Suplazin
Suplasin is a drug prescribed for the symptomatic treatment of osteoporosis.
Available in the form of a disposable syringe bottle.
Contains sodium salt of hyaluronic acid (the main component of synovial fluid) and phosphate buffer.
Thanks to the introduction of Suplazin into the synovial cavities, normal lubrication of the joints is restored, which helps to normalize their general condition and significantly reduce pain symptoms.
Indications.
Osteoporosis.
Conditions after arthrocentnesis, when it is necessary to ensure replacement of synovial fluid.
Application.
The dosage of the drug depends on the size of the joint (2-6 ml).
Typically, 2 ml of Suplasin is injected into the joint for three weeks, one injection per week.
If we are talking about chronic processes, then the duration of treatment can be up to one and a half months.
The drug is administered under aseptic conditions.
Simultaneous treatment of several joints with Suplazin is allowed.
The instructions for Suplazin state that the drug has no contraindications, with the exception of allergies to components or excipients.
If pain, a feeling of heat, swelling or redness occur at the injection site, and if these manifestations do not go away on their own within a few days, it is necessary to consider stopping Suplasin therapy.
Special instructions.
Used syringes with Suplazin and the remains of the drug must be disposed of.
It is not allowed to administer the drug using instruments that were sterilized using a quaternary ammonium hydroxide solution.
Warning
Do not use in patients with known hypersensitivity reactions. The usual precautions and contraindications for intraarticular injections must be observed. Do not administer intravascular.
Suplasin should not be used in patients with inflammation, since it is in such patients that adverse reactions often occur. Since there are no clinical data on the use of hyaluronic acid in children, pregnant and lactating women, treatment with the medical device Suplazin is not recommended for this category of patients. After the injection, the patient should rest for 24 to 48 hours and avoid any strain or physical activity during the entire course of treatment.
Warning: For use by medical professionals only. For one time use only. The unused part of the syringe should be thrown away. Reuse may pose a risk of contamination and/or lead to infection or cross-infection in the patient.
Do not use if blister is damaged.
Lazine®5.0 (Lazine®5.0)
The pharmacokinetic parameters of levocetirizine change linearly.
Suction
After oral administration, the drug is quickly and completely absorbed from the gastrointestinal tract. Eating does not affect the completeness of absorption, although it reduces its speed. The maximum concentration (Cmax) in blood plasma is reached after 0.9 hours and is 270 ng/ml, the equilibrium concentration is reached after 2 days.
Distribution
Levocetirizine is 90% bound to plasma proteins. The volume of distribution (Vd) is 0.4 l/kg. Bioavailability reaches 100%.
Metabolism
In small quantities (<14%) it is metabolized in the body by N- and O-dealkylation (unlike other H1-histamine receptor antagonists, which are metabolized in the liver using the cytochrome system) to form a pharmacologically inactive metabolite. Due to negligible metabolism and lack of metabolic potential, interaction of levocetirizine with other drugs is unlikely.
Removal
The half-life (T1/2) in adults is 7.9 ± 1.9 hours. In young children, the half-life is shorter. In adults, the total clearance is 0.63 ml/min/kg. About 85.4% of the administered dose of the drug is excreted unchanged by the kidneys through glomerular filtration and tubular secretion; about 12.9% - through the intestines.
Selected patient groups
Patients with kidney failure
In patients with renal failure (creatinine clearance (CC) < 40 ml/min), drug clearance is reduced. In hemodialysis patients, total clearance is reduced by 80. Less than 10% of the drug is removed during a standard 4-hour hemodialysis procedure.
Patients with liver failure
Pharmacokinetics in patients with hepatic impairment have not been studied. In patients with chronic liver diseases (hepatocellular, cholestatic and biliary cirrhosis). Those receiving the racemic compound cetirizine at a dose of 10 or 20 mg as a single dose experienced a 50% increase in half-life and a 40% decrease in drug clearance compared to healthy subjects.
Children
Data from a study of the pharmacokinetics of the drug in 14 children aged 6 to 11 years weighing from 20 to 40 kg with a single oral dose of 5 mg of levocetirizine showed that Cmax and area under the curve (AUC) were approximately twice as high as in healthy adults with cross-control. The mean Cmax was 450 ng/ml, the maximum concentration was reached after an average of 1.2 hours, the total body weight-adjusted clearance was 30% higher, and the half-life was 24% shorter in children than in adults. Specific pharmacokinetic studies have not been conducted in children under 6 years of age. A retrospective pharmacokinetic analysis was conducted in 323 patients (181 children aged 1 to 5 years, 18 children aged 6 to 11 years, and 124 adults aged 18 to 55 years) who received one or more doses of levocetirizine 1.25 mg. up to 30 mg. Data obtained during the analysis showed that taking the drug at a dose of 1.25 mg in children aged 6 months to 5 years leads to plasma concentrations similar to those in adults when taking 5 mg of the drug once a day.
Elderly patients
Pharmacokinetic data in elderly patients is limited. When repeated dosing of levocetirizine 30 mg once daily for 6 days in 9 elderly patients (ages 65 to 74 years) total clearance was approximately 33% lower than that in younger adults. The distribution of cetirizine racemate has been shown to be more dependent on renal function than on age. This statement can also be applied to levocetirizine, since both levocetirizine and cetirizine are excreted primarily in the urine. Therefore, in elderly patients, the dose of levocetirizine should be adjusted depending on renal function.
Side effects
After the injection, local reactions may occur, such as short pain in the joint, a feeling of heat, redness of the skin, joint effusion, irritation and swelling, inflammation, hyperthermia. If local symptoms occur, it is necessary to leave the affected joint at rest and apply an ice pack locally. The severity of these symptoms weakens or disappears within a few days in most patients.
In some cases, mainly in the presence of hypersensitivity, local reactions such as pain, irritation, swelling, joint inflammation and joint effusion can be much more pronounced. In such cases, therapeutic intervention, such as aspiration of joint fluid, may be necessary.
Local adverse reactions may be accompanied by systemic reactions such as fever, chills or cardiovascular reactions, and in some cases anaphylactic reactions. In extremely rare cases, rash, itching, urticaria, synovitis and decreased blood pressure have been reported after using Suplazin solution. If the above adverse reactions occur, you must stop using the medical product. It is not recommended to use disinfectants containing quaternary ammonium salts, as sediment may form upon contact with hyaluronic acid.
Suplazin 1-Shot synovial fluid prosthesis 10 mg/ml 6 ml syringe 1 pc
Release form
Synovial Fluid Prosthesis
Package
1 syringe
pharmachologic effect
Restoring the viscoelastic properties of synovial fluid.
Indications
For the symptomatic treatment of osteoarthritis. Suplasin® has shown its benefits in the treatment of osteoarthritis to eliminate pain and improve the physical function of joints.
More than one joint can be treated at the same time.
Contraindications
Do not administer to patients with established hypersensitivity reactions.
Observe the usual precautions and contraindications for any intra-articular injection.
Intravascular injections are prohibited.
special instructions
The patient should remain at rest for 24-48 hours after the injection and avoid any physical activity during the entire course of treatment.
After intra-articular injection, short-term transient pain may occur. The affected joint may exhibit mild local reactions such as pain, burning, overheating, redness, effusion, irritation and swelling/inflammation. If these symptoms occur, keep the affected joint immobilized and apply local ice. For most patients, these symptoms resolve within a few days. In some cases, namely hypersensitivity, mild local reactions such as pain, irritation, swelling/inflammation of the joint and effusion can be significantly longer and more severe. In such cases, therapeutic measures such as aspiration of synovial fluid may be necessary.
Local adverse reactions may be accompanied by general body reactions, such as fever, chills or reactions from the cardiovascular system, and in rare cases, anaphylactic reactions.
Compound
1 syringe (6 ml) contains:
Active substance: sodium salt of hyaluronic acid 60 mg;
Excipients: sodium chloride solution and phosphate buffer solution - up to 6 ml.
Directions for use and doses
Suplazin 1-SHOT, 6 ml is intended for single administration.
The patient should remain at rest for 24–48 hours after the injection and avoid vigorous physical activity during the entire course of treatment. After intra-articular injection, a mild local reaction may occur: short-term pain, fever, feeling of heat, redness, joint effusion, irritation, swelling/inflammation. If these symptoms occur, it is recommended to unload the affected joint and apply local ice. Typically, these symptoms go away on their own within a few days.
Injection technique
Remove the blister with the syringe from the cardboard packaging. Make sure there is no damage or loss of integrity of the blister. Open the blister by holding the paper film by the “tab”.
Remove the rubber cap of the syringe adapter and connect it to a needle of the required caliber (the needle must be prepared in advance taking into account the patient’s characteristics; a recommended needle is 21–25 G, depending on the joint).
Make sure the needle/adapter connection is secure. The graduation on the syringe is used only as an indicator of volume.
Administer the drug according to standard intra-articular injection practice. For ease of administration of the drug, the syringe is equipped with a finger rest. The piston stroke is smooth, without jerking.
Storage conditions
At a temperature of 4–25 °C. Do not freeze!
Best before date
3 years
Possible product names
- Suplasin 1-shot synovial fluid prosthesis 10 mg/ml 6 ml syringe 1 pc.
- Suplasin 1-shot synovial fluid prosthesis syringe 6ml No. 1