Pharmacology
Participates in tissue regeneration. Designed for external and internal use. Chondroxide has a complex effect:
- prevents the development of osteochondrosis and osteoarthrosis;
- able to eliminate painful sensations;
- eliminates inflammatory processes;
- starts the processes of restoration of cartilage tissue;
- makes joints more mobile;
- removes swelling of joints.
How to take it correctly
The tablets must be taken orally twice a day. Take a small amount of liquid. Initially, the duration of the therapeutic course averages 5-6 months. Upon completion of taking the drug, the result lasts for approximately the same time, taking into account the severity of the condition.
Topical medicine (gel, ointment) is applied three times a day. The gel should be applied to the affected area, rubbing in smooth movements until completely absorbed. The therapeutic course can last from 2 weeks to 3 months.
You can use the ointment for no longer than 3 weeks. The product should also be rubbed into the skin with smooth movements.
Taking into account the patient’s condition, the attending physician may prescribe another course of therapy.
The use of chondroxide ointment in the treatment of patients with spinal osteochondrosis
O. N. Shchepetova
The purpose of the study was to study the effectiveness of phonophoresis therapy with chondroxide ointment, used in a complex of standard rehabilitation measures in patients with spinal osteochondrosis. A special feature of the work was the use of ointment using phonophoresis.
The objectives of the study included studying the effect of this drug on the severity of pain, the dynamics of clinical manifestations, and identifying the nature and frequency of side effects.
Material and research methods
The study was conducted at the Rehabilitation Center of the Nizhny Novgorod Research Institute of Traumatology and Orthopedics.
Two groups of patients were studied: the main group and the control group. In patients of the main group, phonophoresis with chondroxide ointment was included in the complex of standard treatment measures. Patients in the control group received only a complex of standard therapy.
The criteria for inclusion of patients in the study were: patient age from 18 to 70 years, diagnosis of spinal osteochondrosis of stages I-II, accompanied by pain and limitation of functional activity. Exclusion criteria were: the presence of a herniated disc, inflammatory rheumatic diseases, active tuberculosis, systemic connective tissue diseases, severe concomitant pathology with decompensation of cardiac, respiratory, and renal activity.
The main group consisted of 21 patients (7 men and 14 women) aged from 18 to 59 years. The average age of men was 44.0±6.9 years, women - 34.9±3.6 years. Lesions of the cervical spine were in 4 patients, thoracic - in 5, lumbar - in 12. Clinically, this manifested itself in the form of syndromes of cervicalgia, thoracalgia and lumboischialgia; all patients had impaired motor activity.
The control group included 10 patients (five men and five women) aged from 33 to 60 years. The average age of men was 49.0±2.2 years, women - 43.4±5.2 years. In 2 patients there was involvement of the cervical spine, in 3 - in the thoracic region and in 5 - in the lumbar region. Patients of both groups were undergoing inpatient or outpatient treatment at the specified Center. In all patients, the diagnosis of spinal osteochondrosis was confirmed by X-ray data. The duration of the disease ranged from 1 year or more.
At the beginning of treatment and upon discharge, the patient’s clinical condition and severity of pain were assessed. The severity of pain was assessed by the patient himself using a visual analogue scale (VAS) for pain [4]. We regarded values on a scale from 1 to 3 as mild pain syndrome, from 4 to 6 as moderate and from 7 to 10 as severe. To assess the clinical condition of patients, we used the scheme proposed by I.N. Morozov [2], which allows you to assess pain, motor activity, the severity of scoliosis and tension symptoms; the maximum number of points on this scale—15—corresponded to the maximum severity of neurological symptoms.
The effectiveness criteria were the presence and severity of pain during palpation, at rest, and during movement; degree of severity of scoliosis and tension symptoms; mobility in the spine; severity of pain and discomfort according to the visual analogue scale.
The effect was considered pronounced when a clinically significant improvement in subjective symptoms was achieved, tension symptoms disappeared, and mobility in the spinal segments was restored. Positive dynamics of subjective symptoms, a decrease in the severity of tension symptoms and a slight increase in motor activity were regarded as a significant improvement.
Based on the obtained quantitative results, a special formula was used to calculate the coefficient of dynamics (DC), reflecting the quantitative (how much a given parameter has changed over a certain period of time) and qualitative change in the analyzed parameter by the end of the course of rehabilitation treatment relative to its beginning, and the trend of development of the symptom during the study was determined .
The results obtained were processed using variation statistics methods [3]. To compare the average trends in the indicators of the main and control groups, the paired Wilcoxon test was used for related samples, and when comparing the dynamics coefficients, the Wilcoxon-Mann-Whitney test was used for unrelated pairwise samples [1].
All patients received drug therapy, which included the use of painkillers and anti-inflammatory drugs (movalis, ketonal, xefocam), drugs that improve microcirculation (actovegin, nicotinic acid, reopoliglucin), reduce muscle tension (sirdalud, mydocalm), antidepressants (coaxil) and vitamins (milgamma ). In addition, various methods of physiotherapy, massage and therapeutic exercises were used simultaneously.
In accordance with the objectives of the work, the treatment of patients in the main group, along with the treatment methods listed above, included the administration of phonophoresis with chondroxide ointment from the 5th-6th day. The procedure was performed on the UZT.1.01 device (state registration # 94/271-100). They were exposed to ultrasound with an intensity of 0.2-0.4 W/cm2 in a pulsed mode using a labile technique for 8-10 minutes. The average number of procedures was 12-15.
Chondroxide ointment produced by JSC Nizhpharm (Russia) was used, which contains 5.0 g of chondroitin sulfate and 10.0 g of dimethyl sulfoxide per 100 g, a substance that promotes deeper penetration of the drug. The use of other systemic and local chondroprotectors (except chondroxide ointment) and hormonal drugs during treatment was considered unacceptable.
results
The severity of pain in patients of the main group at the time of admission to treatment was within the range of 5-10 according to VAS, in patients of the control group - 6-10 (there were no significant differences). In this case, the maximum assessment values occurred in patients with lesions of the lumbar spine. The average pain ratings on the visual analogue scale in both groups also did not differ significantly, amounting to 7.8±0.3 and 7.9±0.4, respectively (p>0.05). Analysis of the results of the study of the clinical condition of the patients also did not reveal any difference in the main and control groups before the start of therapy, amounting to 9.8±0.7 and 9.3±1.1, respectively (p<0.05). This indicates the same severity of pathology in the study groups.
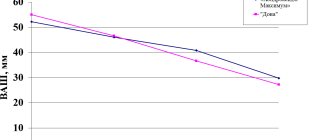
At the end of the course of rehabilitation treatment, patients in both groups experienced regression of pain, symptoms of tension disappeared or significantly decreased, and movements in the spinal segments were restored (see table).
VAS ratings of pain by patients in the main group ranged from 0 to 3, and in the control group - from 0 to 5. At the same time, the average values of patient assessment of the severity of pain were significantly lower (p <0.05) in the main group (0.90 ±0.18) than in the control group (2.4±0.5).
In addition, there were significant differences in the results of assessing the clinical condition of patients on four test scales (pain, tenderness on palpation, impaired motor activity and tension symptoms). In patients of the main group, the average severity of these symptoms was significantly lower. Only on the “scoliosis” scale there were no significant differences. In our opinion, this is explained by the fact that the cause of the development of thoracic osteochondrosis in three patients of the main group was kyphoscoliosis, the anatomical state of which could not change during treatment.
Calculation of the Wilcoxon test confirmed the general positive trend in changes in the studied parameters in patients of the main and control groups on all scales (p<0.0005), except for the “scoliosis” subscale (p<0.01). The general positive trend in changes in pain parameters, tension symptoms, and range of motion in the group of patients receiving phonophoresis with chondroxide ointment was more pronounced. Analysis of the dynamics coefficients using the Wilcoxon-Mann-Whitney test revealed a greater degree of change in pain parameters according to VAS (p<0.002) and on the pain syndrome scale (p<0.004). Changes in other parameters in the main and control groups occurred approximately equally. The coefficient of dynamics of the total score when assessing the clinical condition in the main group was -82.6%, in the control group -69.9% and had significant differences according to this criterion (p <0.05).
It should be noted that no complications or adverse reactions were registered during the use of phonophoresis with chondroxide ointment.
Patients' assessments of treatment results in the main group were generally higher than in the control group. Thus, patients in the main group rated the treatment result as “marked improvement” in 16 cases and as “significant improvement” in 5. In the control group, the treatment result was rated as “marked improvement” by only 2 patients, as “significant improvement” by 6 and as “minor improvement” - 2. Doctors’ assessment of the achieved outcomes in the main group did not always coincide with the patient’s self-assessment: the attending physicians rated the treatment result as “marked improvement” in 14 cases and as “significant improvement” in 7. In patients in the control group, outcome assessment treatment by doctors and patients coincided.
Thus, as a result of the study, it was found that the inclusion of phonophoresis of chondroxide ointment in the program of rehabilitation measures for patients with stage I-II spinal osteochondrosis contributes to more rapid relief of pain, the disappearance of tension symptoms, and the restoration of motor activity of patients. The use of phonophoresis with chondroxide ointment in patients with spinal osteochondrosis is safe and does not cause side effects and can be recommended for inclusion in a complex therapy.
Literature
1. Gubler E.V. Computational methods for analysis and recognition of pathological processes. M: Medicine 1978; 294. 2. Morozov I.N. Restorative treatment of patients after surgical removal of herniated intervertebral discs of the lumbar spine: Dis. ...cand. honey. Sci. N. Novgorod 2001; 183. 3. Tukshaitov R.Kh. Methodological aspects of increasing the information content of the biometric indicator of the significance threshold. Kazan Med Journal 1999; 80:4:259-261. 4. Fishman B., Pasternak S., Wallenstein Sl. et al. The Memorial Pain Assessment Card: A valid instrument for the evaluation of cancer pain. Cancer 1987; 60: 24-28.
Source Journal of Neurology and Psychiatry named after. S.S. Korsakov No. 4 | 2004
Contraindications
The medicine in tablet form should not be taken if:
- bearing a child;
- during breastfeeding;
- children;
- personal intolerance to constituent elements.
External agents should not be taken:
- children;
- with excessive sensitivity to the constituent components;
- if the skin is damaged in the area where the product is applied.
Analogs
Cheap but effective analogues of tablets include Chondroitin - Akos. More expensive are Structum and Teraflex. Analogues of the ointment are Arthro-Active and Chondroitin.
Treaflex. The price starts from 350 rubles. This is a drug that is prescribed for osteochondrosis and osteoarthritis. Can be given to children from 15 years of age. Contraindicated during pregnancy and breastfeeding.
Structum. The price of the drug is 1400 rubles. Has anti-inflammatory and regenerating effects. Contraindicated during pregnancy, lactation, and children under 15 years of age.
Chondroxide 5% 30g gel
pharmachologic effect
Tissue regeneration stimulator.
Composition and release form Chondroxide 5% 30g gel
Gel – 100 g:
- Active substance: Chondroitin sulfate - 5 g;
- Excipients: Dimethyl sulfoxide, propylene glycol, isopropanol, ethanol (ethyl alcohol 95%), sodium disulfite (sodium metabisulfite), nipagin, nipazole, carbopol, orange flavor identical to natural, purified water - up to 100 g.
Gel for external use 5% in aluminum tubes of 20, 25, 30, 35, 40 g. The tube along with instructions for medical use of the drug in a cardboard box.
Description of the dosage form
Yellowish translucent gel with a fruity odor.
Directions for use and doses
Externally.
Chondroxide® gel is applied 2-3 times a day to the skin over the lesion and rubbed in for 2-3 minutes until completely absorbed. The course of treatment is from 2–3 weeks to 2–3 months. If necessary, the course of treatment is repeated.
Pharmacokinetics
Chondroxide® gel is well absorbed. In accordance with data obtained in experimental studies on mice using radiolabeled ZN-chondroitin sulfate, the absorption rate of chondroitin sulfate is 14%. Dimegyl sulfoxide promotes better penetration of chondroitin sulfate through cell membranes deep into tissues. After applying the drug Chondroxide® gel to the skin, chondroitin sulfate quickly and selectively enters the joint, reaching a maximum concentration after 30 minutes and subsequent two-phase removal of the drug from the cartilage tissue. Completion of the rapid elimination phase occurs 1 hour after application. The retention time of the drug in the joint is 5 hours.
Indications for use Chondroxide 5% 30g gel
Treatment and prevention of osteoarthrosis of peripheral joints and osteochondrosis of the spine.
Contraindications
Hypersensitivity to the components of the drug.
Carefully
Pregnancy, lactation, childhood (efficacy and safety have not been established).
Use of Chondroxide 5% 30g gel during pregnancy and breastfeeding
Chondroxide® in gel and ointment dosage form should be used with caution and after consulting a doctor during pregnancy and lactation (breastfeeding).
Chondroxide® in gel and ointment dosage form should be used with caution in pediatric patients (efficacy and safety have not been established).
special instructions
Avoid contact of the drug with mucous membranes and open wounds.
If it gets on skin or clothing, the gel is easily washed off with water, leaving no traces.
Use in pediatrics
The drug should be used in children with caution and after consulting a doctor (the effectiveness and safety of use in pediatrics have not been established).
Overdose
Currently, no cases of overdose of Chondroxide® have been reported.
Side effects Chondroxide 5% 30g gel
Possible: allergic reactions.
Drug interactions
Drug interactions with Chondroxide® have not been described.