Medication relief of an acute attack of gout
Gout is a disease associated with a disorder of purine metabolism, characterized by an increase in uric acid in the blood (hyperuricemia) and the deposition of urate in articular and/or periarticular tissues, kidneys and other organs [1–3].
Gout affects about 0.1% of the population. These are predominantly middle-aged or older people (80–90%) with a history of asymptomatic hyperuricemia for 20–30 years. It has been found that men suffer from gout 20 times more often.
The European League Against Rheumatism (EULAR) recommends that uric acid (UA) levels greater than 360 µmol/L (6 mg/dL) be considered hyperuricemia. The causes of hyperuricemia may be obesity, arterial hypertension, taking medications (diuretics, small doses of acetylsalicylic acid, aminophylline, diazepam, diphenhydramine, dopamine, drugs containing caffeine, vitamins B12 and C, lead), genetic defects, and alcohol consumption.
The accumulation of UA in the blood can be due to either its high production (increased synthesis of endogenous purines), or low excretion, or a combination of these mechanisms. Primary overproduction is associated with defects in the enzymatic system of UA synthesis (insufficiency of hypoxanthine-guanine phosphoribosyltransferase and increased activity of ribose phosphate pyrophosphokinase). Secondary hyperproduction – with increased cell breakdown during hemoblastosis, paraproteinemia, chronic hemolysis, chemotherapy. Hyperuricemia often accompanies psoriasis [4].
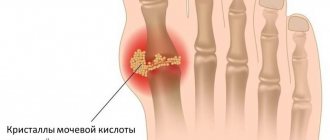
As a result of crystallization of uric acid, microtophi (clusters of crystals) are formed in the synovium and cartilage. Due to injury, increased temperature in the joint, or changes in the concentration of uric acid in the blood or synovial fluid, the microtophi are destroyed and the crystals enter the joint cavity. Synovial cells produce cytokines (interleukins 1, 6, 8 and tumor necrosis factor alpha), which act as chemoattractants for neutrophils. Immunoglobulins and complement components opsonize urates, stimulating the phagocytic activity of neutrophils.
A decrease in urate excretion can be induced by the crystallization of urates in the kidneys against the background of an increase in their excretion (more than 800 mg/day) during primary hyperproduction of urate. As a result, urate tubulointerstitial nephritis develops [5].
The clinical picture of gout is the presence of tophi, damage to the joints and kidneys (interstitial nephritis and nephrolithiasis). It is known that gout has a significant impact on the development of the atherosclerotic process and, consequently, cardiovascular diseases, which determines the prognosis of this disease [6, 7].
The debut of gout is considered to be the first attack of acute gouty arthritis, although hyperuricemia can often be observed for a long time before its development, which contributes to the development of nephrolithiasis [8, 9].
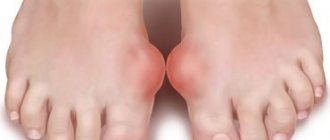
Acute gouty arthritis is triggered by injury, physical activity, sauna use, emotional stress, diet changes (both overeating and fasting), drinking alcohol, infection, surgery, and the use of medications (most often thiazide diuretics, chemotherapeutic anticancer drugs). At the onset of the disease, damage to one joint of the lower extremities is often observed, and in 50% of patients the first metatarsophalangeal joint (MTPJ) is affected.
Gout attacks often occur at night and occur with a rapid increase in erythema and temperature in the joint area, swelling and pain. Inflammation can spread to soft tissues, forming a clinical picture of inflammation of the subcutaneous tissue or phlebitis. There is an increase in body temperature to febrile levels. The usual duration of an attack is from several days to several weeks. In this case, the joint is not deformed.
In a clinical blood test, leukocytosis with a neutrophilic shift to the left and an increase in ESR are determined in patients during acute attacks of gout. In the blood serum there is an increased content of sUA (in men more than 7 mg% (0.42 mmol/l), in women more than 6 mg% (0.36 mmol/l)).
A study of UA excretion should be carried out after a three-day diet excluding purines (meat, broths, fish, poultry, legumes, tea, coffee, cocoa, alcohol, beer). The volume of daily urine, pH, concentration of UA and creatinine in urine and blood serum are determined. Normally, 300–600 mg (1.8–3.6 mmol/l) of SUA are excreted per day.
When analyzing synovial fluid obtained from the affected joint, an increased content of leukocytes (up to 10–60 × 109/l) with a predominance of neutrophils is detected. Of diagnostic significance is the presence of needle-shaped urate crystals located intracellularly and birefringent light when examined using a polarizing microscope.
An X-ray examination of the joints of the feet in the case of chronic gouty arthritis reveals marginal bone or cyst-like formations of regular shape with clear, sometimes sclerotic contours (piercer symptom). Over time, pronounced destruction is observed not only in the subchondral area of the bone, but also in the epiphysis and even in the diaphysis, and intra-articular osteolysis develops. Typically, these changes are most pronounced in the joints of the feet (primarily in the joints of the big toe) [10].
Currently, the most widely used criteria for gouty arthritis are those proposed by S. Wallace et al. and approved by the World Health Organization (2000) [11]:
A. The presence of characteristic monosodium urate crystals in the synovial fluid.
B. Confirmed tophi (chemical analysis or polarization microscopy).
C. Presence of 6 out of 12 clinical, laboratory and radiological signs:
- maximum inflammation of the joint on the first day;
- having more than one attack of arthritis;
- monoarthritis;
- redness of the joints;
- pain and inflammation of the first PFJ;
- asymmetric inflammation of the PFJ;
- unilateral damage to the tarsal joints;
- suspicion of tophi;
- hyperuricemia;
- asymmetric inflammation of the joints;
- subcortical cysts without erosions on X-ray examination;
- absence of microorganisms in the culture of synovial fluid.
It should be noted that in 95.5% of patients with gout, five or more signs are observed already at the early stage of the disease.
Treatment of gout involves a complex of non-drug and medicinal methods aimed at reducing the level of sUA in the blood and preventing damage to various organs and systems. For gouty arthritis, therapeutic nutrition is prescribed (table No. 6): foods containing large amounts of purines are excluded, the consumption of sodium and fat is limited, alkaline mineral waters and citrus fruits are added (to increase the excretion of urates from the body). The total amount of free fluid increases to 2.5 liters, if there are no contraindications from the cardiovascular system.
In the absence of contraindications, the drugs of choice are nonsteroidal anti-inflammatory drugs (NSAIDs) in full therapeutic doses: indomethacin (25–50 mg four times a day), naproxen (500 mg twice a day), diclofenac (25–50 mg four times a day) , nimesulide (100 mg twice daily), etoricoxib (Arcoxia) (120 mg daily). If NSAIDs are ineffective or there are contraindications (for example, treatment with warfarin), colchicine is used (0.5–0.6 mg orally every hour until acute arthritis resolves). This drug should not be prescribed to patients with severe kidney damage, diseases of the gastrointestinal tract, or cardiovascular system, as the risk of severe side effects increases. An absolute contraindication for the use of colchicine is a combination of renal and hepatic failure, a marked decrease in glomerular filtration rate and extrahepatic biliary obstruction.
If it is impossible to use NSAIDs or colchicine, glucocorticosteroids (GCS) are prescribed in the following forms: prednisolone at a dose of 40–60 mg on the first day (tablet form), followed by a dose reduction of 5 mg every other day; triamcinolone – 60 mg IM, if necessary, administration can be repeated after 24 hours; methylprednisolone - 50–150 mg intravenously, in severe cases, small pulse therapy is used: 250–500 mg once, if necessary, the administration can be repeated after 24 hours.
It should be noted that the use of corticosteroids (IV or IM) for gout can cause rebound syndrome and side effects, which requires the patient to stay in the hospital. That is why simultaneous administration of small doses of colchicine (1–2 mg/day) is considered justified [9].
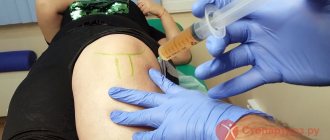
For local injection therapy of acute gouty arthritis, periarticular or intra-articular (with the mandatory exclusion of septic arthritis) administration of GCS is used.
Characteristics of GCS used for local injection therapy of acute gouty arthritis are presented in Table. 1. Hydrocortisone acetate (short-acting corticosteroids) is available in the form of a microcrystalline suspension in 5 ml bottles containing 125 mg of the drug. In terms of severity and duration of action, it is significantly inferior to long-acting corticosteroids, and therefore in recent years it has been used less and less - mainly for infiltration of periarticular tissues or for mild signs of synovitis.
Kenalog 40 is an aqueous crystalline suspension of synthetic fluorinated corticosteroids (triamcinolone acetonide). Available in ampoules of 1 and 5 ml at a concentration of 40 mg/ml. The anti-inflammatory effect occurs one to three days after intra-articular injection and lasts on average up to one month. It should be noted that the drug more often than other corticosteroids causes atrophy of the skin and subcutaneous fat, necrosis of tendons, ligaments, and muscles.
Diprospan® is a prolonged dosage form of betamethasone, a fluorinated derivative of methylprednisolone. Available in 1 ml ampoules containing 2 mg of betamethasone disodium phosphate and 5 mg of betamethasone dipropionate. The first component (highly soluble, quickly absorbed ester) ensures a rapid onset of effect, and the second (poorly soluble, slowly absorbed depot fraction) provides a prolonged effect. Thanks to this combination, the effect of Diprospan begins within 2-4 hours after intra-articular injection and lasts up to three weeks. Another advantage of the drug is that the suspension crystals are micronized. Thus, the concentration of crystals in 1 ml of Diprospan is 6.4 mg/ml, while in 1 ml of Kenalog it is 40 mg/ml. In addition, the average size of Diprospan crystals is from 2 to 6 microns, while the size of Kenalog 40 crystals is about 12 microns. As a result, extra-articular injections are practically painless and are not accompanied by complications. This allows Diprospan to be used without anesthetic.
Our own experience with the use of Diprospan and Kenalog 40 in patients with gouty arthritis showed a higher effectiveness of the former. The use of Diprospan made it possible to reduce the dose of NSAIDs taken and thereby reduce the risk of developing NSAID gastropathy [9].
Consequently, local use of Diprospan has great advantages over other GCS.
According to the EULAR recommendations for the diagnosis and treatment of gout, intra-articular injections of long-acting corticosteroids are the safest and most effective. The dosage and frequency of their administration into large, medium and small joints are presented in table. 2.
A promising method for relieving an acute attack of gout is the use of biological therapy. A study of the effects of anakinra and canakinumab, recombinant soluble antagonists of human interleukin 1, suggested that blocking interleukin 1 may have clinical significance in this pathology [9, 10]. In particular, with subcutaneous administration of the drug at a dose of 300 mg per day (injections were repeated three times), rapid relief of a gout attack was noted. No negative effects were observed. Consequently, in case of ineffectiveness of traditionally used drugs (NSAIDs, colchicine, corticosteroids), the use of biological drugs seems possible.
Thus, early recognition of gouty arthritis and correct medication tactics will help preserve patients’ ability to work for many years and improve the prognosis of the disease.
How is an onset of illness recognized?
Remember: your health is of much greater interest to you than to the most conscientious physician. If there are any violations, you yourself must make an appointment with a doctor, review the examination results, and accept the recommendations. The doctor has many patients, and you have only one (one), so monitor your health personally. The earlier the diagnosis is made, the less likely it is that the disease will go into the acute stage, causing you excruciating pain, destroying your joints, and overloading your kidneys.
No self-medication!
The diagnosis has been made - what next?
First of all, listen to doctors, not charlatans. Gout can neither be cured nor slowed down by spells or fumigation from charcoal. And some plants (sour berries, citrus fruits, mustard and other seasonings) can provoke an exacerbation.
All medications are prescribed only by the supervising doctor, whose computer reflects all your chronic diseases, medications taken for other diseases, etc. For example, asthmatics cannot take Voltaren orally, but for external treatment it is possible.
In the acute stage, physiotherapy, mineral waters, mud applications and many other forms of treatment are contraindicated. And even if there is no exacerbation and there has not been one, you should go to the resort with a card containing the results of your blood and urine tests.
It is necessary to take into account concomitant diseases at the resort. No treatment with baths and massages at elevated temperatures, with caution in case of epilepsy, varicose veins, cardiac and bronchial asthma. Such spa therapy is strictly contraindicated for nephritis, nephrosis, heart disease, during pregnancy and lactation, and mental disorders. You should definitely inform your spa doctor about all these processes, and they will find something alternative for you.