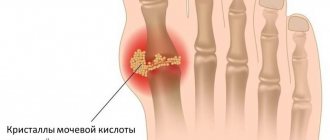
Gout is a disease associated with impaired metabolism of special nitrogen-containing substances - purine bases. Purine bases are part of nucleic acids - DNA and RNA, which are responsible for the storage, transmission and implementation of hereditary information in each cell. With gout, the end product of purine base metabolism, uric acid, accumulates in the body.
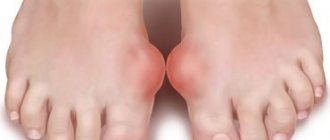
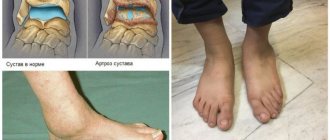
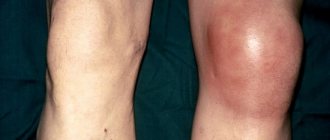
Uric acid in excess amounts is deposited in the form of crystals, most often with the formation of gouty nodes (tophi) in small joints, causing their inflammation (gouty arthritis), as well as in the kidneys, which leads to the development of urolithiasis. In later stages, uric acid crystals are deposited in other organs and tissues (especially in cartilage, near joints).
Gout is accompanied by severe pain in the affected joints (usually the small joints of the toes or hands), which occurs in the evening, intensifies at night and subsides in the morning. In this case, there may be an increase in temperature - fever.
To treat gout, a special group of drugs is used, called anti-gout drugs.
Classification of drugs for the treatment of gout
Antigout drugs are classified into:
- drugs that inhibit the formation of uric acid (uricodepressants): allopurinol, febuxostat;
- drugs that increase the excretion of uric acid (uricosuric drugs): citric acid + trisodium citrate + potassium bicarbonate, sulfinpyrazone, benzobromarone, probenecid;
- anti-inflammatory drugs: colchicine, glucocorticosteroids (prednisolone, triamcinolone, methylprednisolone), non-steroidal anti-inflammatory drugs (diclofenac, indomethacin, naproxen, ibuprofen, ketoprofen, nimesulide).
When and to whom is Colchicine contraindicated? Compatibility with other drugs
Despite the fact that this is one of the best treatments for gout, it has a number of serious contraindications. This is also due to the fact that the active ingredient colchicine is an alkaloid and such “combinations” can cause serious damage to organs and tissues.
The best remedy for gout is Colchicine
UNDER NO EVENT should you take it together with erythromycin, cyclosporine clarithromycin, or calcium channel blockers! This can lead to the most dire consequences. Its combination with Digoskin, statins and fibrates is prohibited. It is strictly not recommended to drink alcohol.
Treatment with it is also contraindicated for:
- hypersensitivity to alkaloids;
- an allergic reaction to one of the components (and a tendency to allergies in general);
- alcoholism;
- chronic kidney and liver diseases;
- problems related to bone marrow;
- purulent inflammations.
Pregnant and breastfeeding women should also not take these tablets. Elderly people and children under 6 years of age are prescribed the drug with extreme caution. The risk group also includes people with serious gastrointestinal problems, as well as those who suffer from cardiovascular diseases. The drug impairs the body's absorption of vitamin B12.
Gout Treatment Basics
Diet plays a major role in the treatment of gout. If you have gout, you should limit your consumption of foods rich in purines - red meat, legumes, cheese, cottage cheese, chocolate. In addition, you must avoid drinking alcohol (especially beer and wine).
If you have gout, it is important to maintain adequate water balance - drink at least two liters of water per day. Alkaline drinking is especially recommended (a teaspoon of soda per liter of water, alkaline mineral waters), since uric acid is more easily excreted in the alkaline environment of urine. In an acidic environment, uric acid precipitates in the form of crystals and is deposited in the kidneys.
Colchicine, glucocorticosteroids and non-steroidal anti-inflammatory drugs are used for an acute attack of gout.
During the interictal period, to reduce the level of uric acid in the blood and prevent gout attacks, drugs that inhibit the formation of uric acid - allopurinol and febuxostat - are used.
A combined drug containing citric acid, trisodium citrate and potassium bicarbonate is prescribed for urolithiasis to alkalize urine to facilitate the dissolution and removal of urinary stones.
Gout pills sir
Despite the successive economic crises, deteriorating environmental conditions and decreasing quality of products, life expectancy in most countries of the world is growing. We begin to live longer, and eat tastier and more varied than just half a century ago. It is these trends that are associated with the increase in the prevalence of gout.
Over the past 30 years, people have become twice as likely to suffer from the disease, the cause of which was determined even before our era by the famous physician Galen, who said that incontinence was to blame. Followers of Gargantua and Pantagruel, connoisseurs of tasty but not very healthy food, doom themselves to the risk of developing chronic arthritis, which is characterized by periodically recurring extremely painful attacks of inflammation, most often in the joint at the base of the thumb.
Like on broken glass
The immediate cause of the inflammatory process in gout is the accumulation of sharp uric acid salts and urates in the joint, like glass fragments. They physically damage the joint membrane, which is accompanied by excruciating pain, swelling and redness in the affected area.
The trigger, i.e. the trigger, for the development of an attack is often a hearty lunch or a hearty dinner. It is with foods, in particular meat, fish, coffee, tea, alcohol (especially beer), that purines enter the body, as a result of the decomposition of which uric acid is formed.
Gout almost always comes at night, suddenly. Overtaking a lover of delicious food in bed, it often does not allow him to leave the bedroom for several days - the pain is so severe. After the attack ends, usually after 5–7 days, the uric acid level returns to normal, and gout goes away on its own for a period of time.
Fortunately, in our time, there is no need to wait a week, languishing in excruciating pain in the tight fetters of gout, because there are drugs that can quickly improve your well-being.
And again NSAIDs
Gout is arthritis. And any inflammatory disease of the joints is treated primarily with the help of non-steroidal anti-inflammatory drugs. They do not affect the level of uric acid, but they allow you to quickly and effectively relieve pain and reduce the severity of inflammation.
The group of NSAIDs is extensive. And although all its representatives have both anti-inflammatory and analgesic properties, in case of an acute attack of gout, preference is given to those drugs that exhibit a powerful and rapid analgesic effect: naproxen, ketoprofen, ibuprofen, diclofenac and indomethacin. To achieve optimal results, the maximum dosage of drugs is prescribed for 10–14 days, depending on the response to therapy.
It should be remembered that while taking NSAIDs, gastrointestinal side effects may develop, and therefore for people suffering from peptic ulcers, gastritis and other gastrointestinal diseases, drugs from this group may be contraindicated.
Uricosuric drugs
Uricosuric drugs block the reabsorption of uric acid in the renal tubules, resulting in a decrease in its level in the blood. But while the urate content in the joints decreases, on the contrary, there is more of it in the urine (they are simply filtered from the blood into the urine). And this is already associated with the risk of stones and the development of kidney stones.
Uricosuric drugs include colchicine and probenecid. They are included in international recommendations for the treatment of an acute attack of gout. In order to prevent the formation of stones, colchicine and probenecid are prescribed in short courses and in low doses. However, Russians will not have to worry about the tolerability of uricosuric drugs, since today they are not registered in our country.
Uric acid synthesis blockers
Their name in pharmacology for most consumers sounds very “heavy” - xanthine oxidase inhibitors. However, most gout sufferers know this name by heart and take the appropriate medications.
Xanthine oxidase inhibitors include the well-known anti-gout drug allopurinol, which combines fairly pronounced activity with availability, as well as the much less economical febuxostat. Both the first and second block the enzyme involved in the synthesis of uric acid, which leads to a decrease in the level of urate in the blood.
Xanthine oxidase inhibitors are prescribed for both treatment and prevention of gout attacks (in long courses). However, starting to take drugs in this group can provoke an exacerbation of the disease, so they are not recommended to be prescribed during an acute inflammatory process.
Hit with hormones!
But drugs of adrenal hormones, glucocorticosteroids, unlike xanthioxidase inhibitors, are capable of stopping an acute attack of gout in a short time and very effectively. However, they are not prescribed to everyone and not always - the list of side effects when taking drugs from this group systemically is too long. Among the most common are edema, excretion of potassium, calcium, increased blood pressure, increased blood glucose levels, increased blood clotting, and menstrual irregularities.
To avoid the development of adverse reactions, corticosteroid hormones are prescribed only to those who cannot tolerate NSAIDs and colchicine, and only in short courses. Most often, prednisolone, triamcinolone, and corticotropin are used for this purpose in the form of tablets or injections.
Marina Pozdeeva
Photo depositphotos.com
Products by topic: (naproxen), (ketoprofen), (ibuprofen), [product strict=”diclofenac”](diclofenac), [product strict=”allopurinol”](allopurinol), (febuxostat), [product strict=”prednisolone "](prednisolone), (triamcinolone)
Treatment of gout: problems, paradoxes, prospects
Magazine "Medical Council" No. 2/2020
DOI:
10.21518/2079-701X-2020-2-104-108
Interview with a leading expert in the treatment of gout, head of the laboratory of microcrystalline arthritis of the V.A. Research Institute of Rheumatology. Nasonova” by Candidate of Medical Sciences Maxim Sergeevich Eliseev.
Gout treatment: problems, paradoxes, prospects
Interview with a leading expert in the treatment of gout, head of the laboratory of microcrystalline arthritis of the Federal State Budget Scientific Institution “Scientific Research Institute of Rheumatology named after VA Nasonova“ candidate of medical sciences Maxim Sergeevich Eliseev.
- Maxim Sergeevich, what can you say about the significance of the problem of gout at the present time? What are the incidence trends in Russia and in the world?
– Gout is currently understood as a disease that is manifested by acute attacks of arthritis due to the deposition of monosodium urate crystals in the joints against the background of increased levels of uric acid. However, it should be remembered that crystals can not only be deposited in joints, but also affect various organs and systems, including, for example, blood vessels. Therefore, the problem is not simply to cure acute arthritis, but to cope with the systemic manifestations of the disease, since patients with gout are much more likely to die from cardiovascular diseases (CVD) and suffer from kidney disease. In recent years, a number of studies have been conducted that have clearly highlighted this problem. So, if 20 years ago there were much fewer patients hospitalized with gout than patients with rheumatoid arthritis (RA), today the opposite picture is observed. In the USA, for example, today there are 2 times more patients with gout hospitalized than with RA. Despite the fact that new drugs for the treatment of gout are being developed and actively introduced, a clear breakthrough has not yet been achieved. While the introduction of new drugs has made it possible to achieve significant progress in the treatment of RA, there is no result yet with regard to gout - we are marking time, while the costs of treating these patients are constantly growing.
As for mortality, its rates, again, in RA have decreased significantly and are gradually approaching the population level, while this does not happen in gout. The main reasons are poor control of uric acid levels (one of the main risk factors for kidney disease and CVD) and concomitant diseases, such as arterial hypertension, diabetes mellitus (DM), and lipid metabolism disorders. The bulk of the patients we hospitalize are under 50 years of age, and most of them already have chronic serious illnesses, which determine future unfavorable outcomes.
If we talk about the incidence rate, in developed countries with a high standard of living, the incidence of gout among the adult population ranges from 1 to 6%. In the male population this percentage is even higher. In the UK, for example, it is 2.5%, in the USA – 4%, in Taiwan – 6%. In the Russian Federation, population-based studies of the prevalence of gout have not been conducted, but we can extrapolate data on the prevalence of hyperuricemia, given that on average every fifth patient with hyperuricemia develops gout. So, in terms of the prevalence of hyperuricemia, Russia is approximately in the middle between the UK (13–14%) and the USA (20–21%), this figure in our country is about 17%. If you divide it by 5, it turns out that approximately 3% of the adult population in our country suffers from gout. In most countries where prospective population-based studies are conducted, this figure either continues to increase or remains at the same level. If we consider the absolute number of people suffering from the disease, then in total it increases.
- What do you think causes poor uric acid control?
– I would say that one of the main reasons is the insufficient knowledge of doctors regarding how to treat a patient in order to achieve an effective reduction in uric acid levels. The second point is the low adherence to treatment of the patients themselves, who do not always comply with the doctor’s instructions, even if the treatment is prescribed correctly. Another reason is very poor control of concomitant diseases. For example, in case of kidney disease, treatment should be prescribed as early as possible, and it has certain characteristics. There is a certain paradox. Achieving the so-called treatment goal (targeted therapy principle) in RA can be very difficult; this may require enormous funds and long-term use of expensive drugs, but today rheumatologists are successfully coping with this. And with gout, it is possible to achieve complete control over the disease in 90–95% of cases, but this does not happen. Both doctors and patients have an opinion, not supported by anything, that gout is an incurable disease. Even experts do not believe that progress can be made and the course of the disease can be completely changed. Although with gout there is the possibility of absolute regression of even bone erosions and restoration of bone structure in the presence of intraosseous tophi: deposits of urate crystals can completely resolve there too. And yet, the picture has not yet changed for the better.
Let's imagine a situation: a patient comes to the doctor, his uric acid level was measured, a urate-lowering drug was prescribed, but the level of cholesterol, which is elevated in more than 50% of patients with gout, was not examined, as well as the level of triglycerides (it exceeds the norm in more than 60% of patients ). Diabetes among patients with gout occurs several times more often than in the general population. In some countries its frequency reaches 20%, i.e. Every fifth patient with gout also suffers from diabetes. Earlier diagnosis of these conditions and appropriate treatment could improve the prognosis of the disease, but for some reason this does not happen. The problem is also the choice of the optimal drug, which can both positively and negatively affect the level of uric acid, as well as knowledge of the compatibility of a large number of different medications prescribed in connection with concomitant diseases, including coronary artery disease and heart failure. And you need to be a very competent specialist to choose the optimal treatment strategy.
- Is gout a well-studied disease or are there still some blind spots in understanding its etiology and pathogenesis?
– This disease has been well studied, and this is the second paradox. The first mention of gout is found in the Egyptian Ebers papyrus, which is about 5 thousand years old. That is, gout was described as a disease even before Hippocrates. The basic principles of its treatment were outlined, including treatment with colchicine and the use of special elixirs that could reduce joint pain in patients. A huge number of famous people suffered from gout; Hippocrates and Sydenham, the greatest English doctor of the 17th century, mentioned it. The famous Dutch scientist, inventor of the light microscope and founder of microscopy, Leeuwenhoek, first discovered and described uric acid crystals back in the 17th century. Perhaps this is the oldest joint disease known to us. But, nevertheless, there are many white spots left. It is still unclear why only every fifth patient with high uric acid levels develops gout. Moreover, a linear relationship between the level of uric acid and morbidity is also not observed. It is quite possible, and this is now being studied, that there are genetic factors that are more predisposing to the development of hyperuricemia, and there are those that are identified with a greater likelihood of developing gout, with the crystallization of uric acid salts, without which the disease is impossible. It is not entirely clear how the attack is self-limited. In other words, gouty inflammation develops as part of an autoinflammatory process, i.e. a process associated with the activation of certain Toll-like receptors by urate crystals and, ultimately, the synthesis of proteins that lead to the development of the immune process. It is characterized by the development of acute neutrophilic inflammation, but the mechanisms that lead to self-limitation of this inflammation are not clear.
The role of uric acid has not yet been fully elucidated. A number of studies demonstrate that it is of absolute importance in the development of CVD and kidney diseases, but independently or in connection with crystal deposition is also not entirely clear. Recent work has shown that in patients with elevated uric acid levels but without acute arthritis, you just need to look hard for the crystals, and in most cases they will be identified. And the presence of crystals indicates the implementation of an immune response and, as a result, the development of inflammation. Uric acid in vitro in high concentrations exhibits pronounced pro-oxidant properties and, therefore, can contribute to the development of diseases of the cardiovascular system and kidney pathology. The extent to which these negative effects can be realized in the body’s conditions is not completely clear.
- What risk factors for the disease can be identified?
– They can be divided into two main groups – non-modifiable and modifiable. The first group is genetic factors, primarily those associated with the presence of several urate transporters that ensure the process of reabsorption of uric acid in the kidneys: URAT-1, SLC-2A9, GLUT-9, OAT-4 and others. And to a lesser extent those associated with uric acid excretion (ABCG2). In other words, 90% of patients with gout are people who do not excrete uric acid due to a genetic predisposition. Approximately 75% of uric acid is synthesized in the body itself, and 20–25% comes from outside. The level of uric acid is influenced by diet, lifestyle and a number of diseases, as well as medications (diuretics, some drugs for the treatment of tuberculosis). Modifiable risk factors also include alcohol intake. Among the diseases that can lead to increased uric acid levels are hypertension, heart failure, renal failure, psoriasis, and myeloproliferative diseases. It should be noted that men are more likely to suffer from gout than women, at least before menopause. This is because estrogens have the ability to increase the excretion of uric acid.
- Please tell us how the disease develops and progresses.
– A number of scientists have proposed a sequential, or stepwise, scheme for the development of gout. The first stage is asymptomatic hyperuricemia. It is characterized by increased uric acid levels, the absence of crystals and joint damage. We can call this stage pre-gout. The next stage is the deposition of uric acid crystals against the background of hyperuricemia. How can this be detected? For example, when a patient undergoes routine ultrasound or computed tomography (CT) in connection with another pathology. These methods can detect crystals in 20–25% of patients, especially in the lower extremities. The next stage is hyperuricemia with crystal deposition and the presence of acute attacks of arthritis, that is, gout itself. The disease continues to progress, attacks are repeated, and it ends in severe chronic arthritis with the development of gouty arthropathy, deformation and limitation of joint function, as well as damage to internal organs associated with the deposition of uric acid crystals. It remains unclear to what extent the presence of crystals in patients with elevated uric acid levels increases the risk of developing gout, whether it will inevitably lead to the development of the disease, and when this will happen? On the other hand, if there are no crystals initially, but there is hyperuricemia, what is the probability that gout will not develop? These studies are just beginning to be conducted, and we are unlikely to get answers to these questions in the near future.
- What organs are affected by gout?
– Besides the joints, the main target organs are the cardiovascular system and the kidneys. In almost 100% of cases of gout, deposits of uric acid crystals are found in them. This does not lead to such severe manifestations as crystal deposition in the joint (acute attacks of arthritis, characterized by severe pain), but inflammation that occurs in the vascular wall and in the kidneys develops according to the same laws. And this is probably the main reason why patients with gout die from various cardiovascular accidents much more often than patients without gout. Hypothetically, uric acid crystals can be found in various organs and systems. Thus, in 40% of patients with gout, crystals are found in the prostate during surgery, according to histological examination. It is important to note that gout is currently considered one of the risk factors for prostate cancer.
- What are the features of kidney damage due to gout?
– It is traditionally believed that with gout, the formation of kidney stones necessarily occurs. However, studies have shown that, overall, kidney stones are no more common in patients with gout than in the general population. These are mainly urate stones; phosphate and struvite stones, on the contrary, are less common. On the other hand, those crystals that are deposited in the kidneys, but do not lead to the formation of stones, can cause inflammation in the tubular apparatus. The excretion of uric acid is influenced by urate transporters, the number of which is genetically determined. Normally, 90% of uric acid is reabsorbed by the kidneys, and 10% is excreted. Genetic damage causes this percentage to drop to 5–7. Deposition of uric acid crystals can cause tubulointerstitial inflammation through the same mechanism as in joints. Ultimately, this inflammation can lead to the death of nephrons, renal tubular cells, the development of fibrotic changes, and may also contribute to the development of amyloidosis.
Uric acid can have a negative effect on the renin-angiotensin system. The first studies, still on rats, showed that when uric acid levels increase, the kidneys are the first to suffer. Kidneys suffer and blood pressure rises. We tried to prescribe medications to lower blood pressure - nothing happened. The administration of urate-lowering drugs led to normalization of blood pressure and kidney function. This approach was later used in the treatment of gout in humans. In almost all studies, the administration of drugs that lower uric acid levels led to a statistically significant decrease in blood pressure (BP). Both crystals and uric acid itself in the tubular apparatus of the kidneys can cause the development of inflammation and change the structure of the kidney cells; they become phenotypically different. This leads to decreased function and progression of chronic kidney disease (CKD).
Before they learned to treat gout, that is, before the advent of effective urate-lowering drugs, a significant proportion of patients (up to 20–30%) died from acute urate nephropathy. Today such situations are much less common. When the level of uric acid becomes extremely high, which often happens in myeloproliferative diseases, when cytostatics are prescribed, a huge amount of uric acid accumulates, which simply “clogs” the renal tubules. The only thing that can be done is hemodialysis; previously, alkaline solutions (sodium bicarbonate) were used. Another situation is chronic nephropathy, where chronic inflammation occurs due to the formation of crystals. And here it is difficult to determine what the kidneys suffer from more: high blood pressure, high glucose levels or uric acid.
- What is the prognosis for gouty nephropathy?
– If left untreated, 45–50% of patients with gout will develop clinically significant chronic kidney disease. 10–15% of people suffering from gout are patients with CKD stage 3 or more, i.e. These are candidates for extracorporeal treatment and replacement therapy. This is much higher than in the population and is associated with inadequate treatment of the disease.
- Are there “difficult cases” when diagnosing gouty nephropathy?
– Routinely separating chronic kidney disease associated with hypertension or diabetes from gouty nephropathy may not be easy. Attempts are being made to conduct research using new instrumental capabilities, scintigraphy, and determination of various markers, but, unfortunately, they are not specific for gout. Rather, we are talking about suspecting, even at the preclinical stage, the possibility of developing the problem as such, when it can be influenced. If we see high uric acid levels and, even minimally, a decrease in glomerular filtration rate (GFR), it is advisable to consider the need to prescribe adequate treatment for these patients, regardless of the frequency of arthritis attacks and the presence/absence of urate nephrolithiasis. What does a doctor usually pay attention to? For blood counts, the level of creatinine, which can be used to calculate the glomerular filtration rate, is a more objective indicator reflecting kidney function. Kidney function can be affected by medications, the presence of concomitant diseases, and many other factors. For example, an increase in uric acid levels increases the production of renin, resulting in increased blood pressure, which in turn leads to a deterioration in kidney function and impaired excretion of uric acid. A vicious circle is formed. It is difficult to say what is primary and what is secondary in this chain.
- If we talk about modern standards of treatment for gouty nephropathy, what are the main directions and goals of therapy? What is the criterion for the effectiveness of anti-gout therapy?
– It should be remembered that normally a person loses a certain number of nephrons every year; the kidneys do not recover from damage as actively as the liver. An attempt to influence key risk factors is decisive in the prevention of gouty nephropathy. In other words, if the patient’s blood pressure and glycemic levels are controlled, then nephropathy will not progress. By eliminating these risk factors, we can at least stabilize the condition and possibly improve kidney function for a while. Therefore, research over the past 20 years has been aimed at correcting hyperuricemia as a risk factor for the development and progression of chronic renal failure (CRF). The first works appeared 1.5–2 decades ago and were distinguished by the fact that they were carried out on a limited number of patients and covered a short period of time. But even 6–12 months was enough to talk about the relatively successful use of drugs that reduce uric acid levels. Subsequent studies, primarily with the use of allopurinol, showed that not everything is so obvious. First, the administration of allopurinol did not always slow the progression of CKD compared with control groups. The use of high doses of the drug was limited by the risk of adverse events in patients. We know that allopurinol is largely excreted by the kidneys, as is one of its metabolites, oxypurinol. This may increase the likelihood of adverse events. However, these studies demonstrated that unless uric acid is reduced to a target level at which crystal formation is not possible, little will change globally. The mere fact of taking the drug does not guarantee anything. As in the case of the treatment of other metabolic diseases, we must rely on specific numbers. For example, in diabetes mellitus (DM), the goal is to achieve a certain level of glycated hemoglobin; in patients with hypertension, the goal is to reduce blood pressure. Likewise, with gout, the goal is to reduce the level of uric acid to certain values, the achievement of which will determine the effectiveness of urate-lowering therapy.
If we talk about allopurinol, the drug is effective only in the early stages, when the disease is just beginning. It is known that half of patients with gout have a clinically significantly reduced glomerular filtration rate. In patients with advanced disease, the effect of allopurinol is insufficient. At low doses, the goal cannot be achieved, and high doses cannot be prescribed due to the risk of drug complications. Over the past 10 years, we have begun to receive more and more information about the possibility of using febuxostat, which is able to reduce uric acid levels to a greater extent and more quickly, positively affecting GFR, regardless of the stage of CKD. These studies were longitudinal, allowing for long-term outcomes to be taken into account. During the first two years of taking febuxostat, the average GFR may even increase, which does not happen with allopurinol. The maximum follow-up period currently is 5 years, and the results show that the decline in kidney function in patients taking febuxostat is quite comparable to that in a healthy person with normal uric acid levels. Moreover, this occurs both in patients with gout and with asymptomatic hyperuricemia.
- How is this result achieved?
– By reducing uric acid levels and inhibiting xanthine oxidase, an enzyme that is involved in this process. Inhibition of this enzyme has an effect on oxidative stress by decreasing the production of reactive oxygen molecules and increasing the production of nitric oxide. This is a mechanism for protecting the cell and at the same time reducing the risk of cardiovascular accidents in patients with CKD, since CKD is one of the risk factors for overall cardiovascular mortality in patients with gout, and not only the cause of the development of end-stage renal failure. If we reduce the rate of progression of CKD, cardiovascular risks also decrease, and the patient lives longer.
Studies have been conducted directly comparing allopurinol and febuxostat. Regardless of the severity of kidney damage, febuxostat showed some benefit in both end-stage patients and patients with moderate to severely reduced glomerular filtration rate, i.e. with stages 3, 4, 5 of the disease. In fact, treatment is now carried out in monotherapy mode. Febuxostat may even be effective as an adjunct to hemodialysis. The remaining nephrons work longer, and kidney function remains as preserved as possible. When GFR is below 30, when it is considered that urate-lowering therapy no longer works, prescribing febuxostat even in low doses (up to 40 mg/day) can achieve a good result. At the same time, the drug is very well tolerated.
- What, besides drug therapy, is used today in the treatment of gouty kidney?
– Compliance with a low-salt diet is mandatory. It is necessary to reduce the intake of sodium and potassium and protein. But this is rather prevention, which does not significantly affect the prognosis. Attempts are being made to influence risk factors that, until recently, have not been sufficiently studied. If we lower uric acid levels, we can either prevent or, in the worst case scenario, slow the decline in GFR. Prevention should include complete cessation of smoking, alcohol, and the prescription of diuretics.
- What opportunities do you see for improving disease control?
– If doctors strictly follow the recommendations of the Association of Rheumatologists of Russia and the European Anti-Rheumatic League for the treatment of gout, and patients take the treatment prescribed to them, then, according to our data, which will be published in the near future, the probability of achieving target levels of uric acid can be 95%. With adequate prescription of xanthine oxidase inhibitors and febuxostat, the problem can be solved. In the remaining 5% of cases, drugs with a different mechanism of action, for example uricosurics, peguricase drugs, can potentially help. Another xanthine oxidase inhibitor, topiroxostat, has been developed and used in Japan. There are hypoglycemic drugs that lower uric acid levels, sodium-glucose cotransporter type 2 inhibitors, thiazolidinediones, some lipid-lowering drugs (fenofibrate, rosuvastatin, atorvastatin) and antihypertensive drugs (losartan), although their urate-lowering effect is very moderate. Archalofenate has passed the third stage of clinical trials - it reduces both sugar and uric acid levels, and in addition, has an anti-inflammatory effect.
Many patients refuse to take urate-lowering drugs due to the large number of medications prescribed to them. At a dose of 500 mg/day, you need to take up to 5 tablets of allopurinol per day, and it is necessary to titrate the dose, gradually increasing the number of tablets depending on the level of uric acid. In the case of febuxostat, it is enough to take 1 tablet of 80 mg, and in 2/3 of cases there is no need to increase the dose.
Urate-lowering therapy can not only prevent the development of acute attacks of arthritis, but also reduce the likelihood of developing cardiovascular diseases. If the level of uric acid is below 360 µmol/l, the mortality rate of patients is 2 times less overall, and due to cardiovascular accidents - 2.5 times less. This is proven by the results of a three-year follow-up of more than 1,000 patients with gout, which analyzed the effect of therapy on the risk of overall and cardiovascular mortality, published in 2019.
In addition to controlling uric acid levels, adequate anti-inflammatory therapy is also important. Prescription of the same colchicine in patients with high cardiovascular risk, according to the latest data, reduces the likelihood of developing heart attacks, strokes and thrombosis. That is, colchicine has a double effect - it blocks the development of inflammation both in the joints and in the walls of blood vessels.
An American study examined interleukin-1 inhibitors, which, over long-term follow-up, demonstrated a 15% reduction in the risk of cardiovascular events in patients with both gout and hyperuricemia. Unfortunately, the drug was not registered by the FDA, primarily due to its high cost, and secondly, because the incidence of infectious complications was generally higher than in the population, making its use irrational.
In conclusion, I would like to emphasize once again that adequate use of urate-lowering drugs, primarily xanthine oxidase inhibitors, in patients with gout and asymptomatic hyperuricemia in the presence of CKD can actually slow down the progression of CKD and should be considered as the main direction of treatment.
Interviewed by Lyudmila Golovina
Metabolic syndrome and gout - approaches to antihypertensive therapy
About the article
2953
0
Regular issues of "RMZh" No. 27 dated December 25, 2005 p. 1880
Category: General articles
Authors: Shostak N.A. 1, Anichkov D.A. 1 Federal State Budgetary Educational Institution of Russian National Research Medical University named after. N.I. Pirogov Ministry of Health of Russia, Moscow, Russia
For quotation:
Shostak N.A., Anichkov D.A. Metabolic syndrome and gout - approaches to antihypertensive therapy. RMJ. 2005;27:1880.
Introduction Metabolic syndrome (MS) is a set of cardiovascular risk factors, united by a common pathogenetic mechanism, resistance of peripheral tissues to insulin [1]. The main components of MS are arterial hypertension, abdominal obesity, decreased high-density lipoprotein cholesterol (HDL-C), hypertriglyceridemia and fasting hyperglycemia [2]. At the same time, disturbances in uric acid metabolism, changes in the hemostatic system, endothelial dysfunction, increased levels of C-reactive protein and insufficient reduction in blood pressure at night often accompany MS [3]. These anomalies have pathogenetic, clinical and, in some cases, prognostic significance. Thus, insufficient reduction of blood pressure at night is associated with target organ damage and an increased risk of cardiovascular events in patients with arterial hypertension [4]. Therefore, normalization of the circadian rhythm of blood pressure in patients with MS can be considered as an additional goal of antihypertensive therapy [5].
Metabolic syndrome (MS) is a set of cardiovascular risk factors, united by a common pathogenetic mechanism, resistance of peripheral tissues to insulin [1]. The main components of MS are arterial hypertension, abdominal obesity, decreased high-density lipoprotein cholesterol (HDL-C), hypertriglyceridemia and fasting hyperglycemia [2]. At the same time, disturbances in uric acid metabolism, changes in the hemostatic system, endothelial dysfunction, increased levels of C-reactive protein and insufficient reduction in blood pressure at night often accompany MS [3]. These anomalies have pathogenetic, clinical and, in some cases, prognostic significance. Thus, insufficient reduction of blood pressure at night is associated with target organ damage and an increased risk of cardiovascular events in patients with arterial hypertension [4]. Therefore, normalization of the circadian rhythm of blood pressure in patients with MS can be considered as an additional goal of antihypertensive therapy [5]. Patients with MS belong to the category of high risk of cardiovascular complications [6]. The European Society of Cardiology/European Society of Hypertension guidelines and the 7th report of the US Joint National Committee (JNC-7) recommend all effective and well-tolerated antihypertensive drugs for patients with diabetes mellitus and metabolic syndrome, with an emphasis in JNC-7 on diuretics and b- blockers. However, the effect of various antihypertensive drugs on lipid, carbohydrate and purine metabolism is different. From this point of view, the most justified is the use of modern long-acting ACE inhibitors (in particular, lisinopril). The drug is metabolically neutral [7,8], and according to some data, even increases tissue sensitivity to insulin [9]. It is relevant to study the effect of lisinopril on the level of uric acid in patients with MS. The rationale is based on the results of several modern studies. Hyperuricemia is a risk factor for cardiovascular morbidity [10]. High levels of uric acid increase the risk of the onset and progression of arterial hypertension [11,12]. Uric acid levels are a strong predictor of cardiovascular mortality in middle-aged men, even after controlling for factors often associated with metabolic syndrome and/or gout [13]. Both asymptomatic hyperuricemia and gout are closely associated with insulin resistance and metabolic syndrome. According to PH Dessein et al. (2000), hyperinsulinemia is observed in 95% of people with gout, metabolic syndrome – in 76% [14]. Hyperuricemia is closely related to hypertriglyceridemia [15]. In addition, hyperuricemia can serve as a surrogate marker of insulin resistance syndrome [16]. The possible relationship between hyperuricemia and vascular complications of arterial hypertension is presented in Figure 1. Thus, the purpose of this study was to evaluate the effects of lisinopril (Sinopril) and the comparator drug atenolol on the 24-hour blood pressure profile and the effect of Sinopril on uric acid levels in patients with MS. Atenolol was chosen due to its widespread clinical use and known hemodynamic and metabolic effects. Atenolol is often used as a comparator in clinical trials of antihypertensive drugs [17,18]. Material and methods An open, non-randomized comparative study was conducted in an outpatient setting. Inclusion criteria were: male or female gender; age from 30 to 60 years; a combination of arterial hypertension stages I and II according to the 2003 WHO classification [19] and two other signs of metabolic syndrome according to ATP III criteria [2]; informed consent. Patients were excluded if at least one of the following criteria was present: secondary arterial hypertension, a history of cardiovascular events, arrhythmias, decompensated diabetes mellitus requiring insulin treatment, extreme obesity (BMI>40 kg/m2), for women - pregnancy or lactation. At the screening stage, 41 patients met the inclusion criteria. However, 3 patients refused to participate in the study and 2 patients did not complete the washout period due to a pronounced increase in blood pressure. Thus, the study group consisted of 36 patients with MS. After a one-week introductory (“wash-out”) period, patients were randomly prescribed lisinopril (Sinopril®, JSC Pharm, Russia, together with Eczacıbaşı Pharmaceutical, Turkey) 10 mg or atenolol 50 mg once in the morning. The duration of therapy was 12 weeks. After 4 weeks Blood pressure and heart rate were monitored; if therapy was insufficiently effective, the dose of the drug (lisinopril or atenolol) was doubled while maintaining a single dose regimen. At baseline and after 12 weeks of therapy, a routine clinical examination was performed, including measurement of office blood pressure and heart rate values; anthropometric measurements (height, body weight, waist circumference). Daily blood pressure monitoring was carried out using Meditech ABPM-04 and Schiller BR-102 devices with assessment of average systolic, diastolic blood pressure and heart rate, as well as the degree of nocturnal decrease in blood pressure and heart rate. Patients were advised to maintain a normal activity schedule and keep a diary indicating the events that occurred and the time of night's sleep. Measurements were carried out at intervals of 15 minutes. afternoon and 30 min. at night. Baseline laboratory examination in the Sinopril and atenolol groups included assessment of the serum lipid profile: total cholesterol, triglycerides, high-density lipoprotein (HDL) cholesterol (enzymatic method); fasting serum glucose (oxidase method). In the lisinopril group, all of the above parameters were also assessed after 12 weeks of therapy. Levels of uric acid, potassium and creatinine were assessed at baseline and after 12 weeks of therapy in the Sinopril group only. The main criteria for the effectiveness of therapy were: changes in average daily, average daily and average night blood pressure and heart rate, the degree of nocturnal decrease in blood pressure and heart rate, changes in uric acid levels. Additionally, the proportion of patients who responded to therapy (responders rate) was taken into account, with the response criterion being a decrease in office blood pressure to the target level of 140/90 mmHg. or more than 10% of the initial level. The safety of therapy was assessed by the presence of side effects (no side effects; side effects that do not require discontinuation of the drug; side effects requiring discontinuation) and the absence of unfavorable changes in laboratory parameters (creatinine, potassium). Statistical processing Statistical analysis was performed using the Statistica 6.0 software package (StatSoft Inc., USA). Descriptive statistics methods (mean, standard deviation, median, quartiles, absolute and relative frequencies) were used to present the data. To assess the type of distribution, visual analysis of distribution histograms and the Shapiro–Wilk test were used. Comparison of qualitative data was carried out using Fisher's exact test, quantitative (in the case of normal distribution) - using Student's test for related and unrelated samples. When the distribution of a trait differed from normal, nonparametric methods were used (Wilcoxon and Mann–Whitney tests). The significance level was set to 0.05. Results The initial characteristics of patients in the Sinopril and atenolol groups are presented in Table 1. There were no statistically significant differences between the groups, with the exception of office diastolic blood pressure and pulse rate. The initial indicators of 24-hour blood pressure monitoring were comparable, but there were differences in the average daily and average daily values of systolic blood pressure and heart rate (Table 2). After treatment was prescribed, 1 patient in the Sinopril group was excluded due to an allergic reaction (skin rash). In 2 patients in the atenolol group, the drug was discontinued due to severe bradycardia. Thus, 23 patients in the Sinopril group and 10 patients in the atenolol group completed the study. Sinopril and atenolol equally reduced average daily systolic and diastolic blood pressure (Fig. 2). At the same time, Sinopril reduced systolic blood pressure to a greater extent during the night period, while atenolol decreased systolic blood pressure during the daytime period (Fig. 3). During therapy with atenolol, an expected decrease in heart rate was observed (day: –13.6±1.8 beats/min; daytime period: –15.5±2.0 beats/min., night period: –7.6± 1.1 beats/min., for all indicators p The effect of the studied drugs on the daily rhythm of blood pressure and heart rate is presented in Table 3. In the Sinopril group there were no significant changes in the degree of nocturnal decrease in blood pressure; there was a significant (p = 0.048) increase in the degree of nocturnal decrease Heart rate: In the atenolol group, there was a trend toward a decrease in the degree of nocturnal decrease in systolic blood pressure (p = 0.093) and a statistically significant decrease in the degree of nocturnal decrease in heart rate (p = 0.022).Both drugs showed similar effectiveness in relation to office systolic and diastolic blood pressure (-24 ± 4 /–14±2 mmHg in the Sinopril group and –21±6/–12±2 mmHg in the atenolol group; for all indicators – p Data on the effect of Sinopril on uric acid levels, serum lipids , creatinine and potassium are presented in Table 4. The effect on uric acid levels was positive, but did not reach the level of statistical significance. There was an increase in HDL cholesterol (p=0.018), other parameters did not change significantly. Discussion The choice of an antihypertensive drug in a patient with hyperuricemia and/or gout and MS is very difficult. In addition to high antihypertensive effectiveness and a beneficial effect on the characteristics of the circadian rhythm of blood pressure and heart rate, the drug should not increase the level of uric acid. The main drugs recommended by modern guidelines somehow affect the level of uric acid in the blood serum. Thus, therapy with diuretics is accompanied by an increase in uric acid; during treatment with b-blockers, there is a tendency to increase; at the same time, ACE inhibitors (for example, lisinopril) can reduce uric acid levels [20]. Therefore, the drug of choice in this group of patients may be lisinopril. According to our study, lisinopril (Sinopril), like the comparator drug atenolol, effectively reduces average daily blood pressure in patients with MS. At the same time, the response rate to therapy (according to office blood pressure measurements) in the Sinopril group was 79%, in the atenolol group - only 50%. It is interesting to note that Sinopril reduced nocturnal BP to a greater extent, and therefore favorable changes in the circadian BP rhythm were observed, while atenolol worsened the characteristics of the circadian BP rhythm. The data obtained are consistent with the results of other researchers who studied the effect of lisinopril and atenolol on the circadian rhythm of blood pressure [8,21]. Noteworthy is the positive dynamics of the degree of nocturnal decrease in heart rate in the Sinopril group and negative dynamics in the atenolol group. According to the literature, insufficient reduction of heart rate at night worsens the prognosis in patients with arterial hypertension [22]. Therefore, our results may have clinical implications. Sinopril therapy led to a decrease in uric acid levels, which, however, did not reach the level of statistical significance. The drug increased HDL cholesterol levels, but other indicators remained virtually unchanged. Literary data on the effect of ACE inhibitors on uric acid levels are contradictory. Thus, in a study by DeRosa et al. [23] reported an increase in serum uric acid concentration in patients with arterial hypertension during 3 years of enalapril therapy. On the other hand, there is evidence that lisinopril neutralizes the increased level of uric acid caused by hydrochlorothiazide [24]. The positive effect of lisinopril on serum uric acid levels is due to an increase in uricosuria [20]. Thus, Sinopril has a significant antihypertensive effect (response rate 79%), characterized by normalization of the daily blood pressure profile, an increase in the degree of nocturnal decrease in heart rate and blood pressure, and has a beneficial effect on the lipid profile (increased HDL) and uric acid metabolism. Conclusions 1. Sinopril demonstrates an effect comparable to atenolol on 24-hour blood pressure monitoring. 2. The characteristics of the circadian rhythm of blood pressure do not change under the influence of Sinopril therapy; atenolol adversely affects the circadian rhythm of systolic blood pressure. 3. Sinopril, in contrast to atenolol, increases the degree of nocturnal decrease in heart rate. 4. During Sinopril therapy, there is a tendency for uric acid levels to decrease and HDL cholesterol levels to increase. References 1. Reaven GM. Metabolic syndrome. Pathophysiology and implications for management of cardiovascular disease. Circulation 2002;106:286–8 2. Grundy SM, Brewer Jr HB, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004;109:433–8 3. McFarlane SI, Banerji M, Sowers JR. Insulin resistance and cardiovascular disease. J Clin Endocrinol Metab 2001;86:713–8 4. Ohkubo T, Imai Y, Tsuji I. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens 2002;20:2183–2189. 5. Palatini P, Parati G. Modulation of 24–h blood pressure profiles: a new target for treatment? J Hypertens. 2005;23:1799–1801. 6. Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle–aged men. JAMA 2002; 288:2709–16 7. Thurig C, Bohlen L, Schneider M, et al. Lisinopril is neutral to insulin sensitivity and serum lipoproteins in essential hypertensive patients. Eur J Clin Pharmacol 1995;49:21–6 8. Reisin E, Weir MR, Falkner B, et al. Lisinopril versus hydrochlorothiazide in obese hypertensive patients: a multicenter placebo–controlled trial. Treatment in Obese Patients With Hypertension (TROPHY) Study Group. Hypertension 1997;30(1pt 1):140–5 9. Fogari R, Zoppi A, Corradi L, et al. Comparative effects of lisinopril and losartan on insulin sensitivity in the treatment of non diabetic hypertensive patients. Br J Clin Pharmacol 1998;46:467–71 10. Wang JG, Staessen JA, Fagard RH, Birkenhager WH, Gong L, Liu L. Prognostic significance of serum creatinine and uric acid in older Chinese patients with isolated systolic hypertension. Hypertension 2001;37(4):1069–74. 11. Nagahama K, Inoue T, Iseki K, et al. Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa, Japan. Hypertens Res 2004;27:835–41 12. Sundstrom J, Sullivan L, D'Agostino RB, et al. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension 2005;45:28–33. 13. Niskanen LK, Laaksonen DE, Nyyssonen K, et al. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med 2004;164:1546–51. 14. Dessein PH, Shipton EA, Stanwix AE, et al. Beneficial effects of weight loss associated with moderate calorie/carbohydrate restriction, and increased proportional intake of protein and unsaturated fat on serum urate and lipoprotein levels in gout: a pilot study. Ann Rheum Dis 2000;59:539–43 15. Clausen JO, Borch–Johnsen K, Ibsen H, Pedersen O. Analysis of the relationship between fasting serum uric acid and the insulin sensitivity index in a population–based sample of 380 young healthy Caucasians. Eur J Endocrinol 1998;138:63–9 16. Fam AG. Gout, diet, and the insulin resistance syndrome. J Rheumatol 2002;29:1350–5. 17. UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998;317:713–20. 18. Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomized trial against atenolol. Lancet 2002;359:995–1003. 19. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21(11):1983–92. 20. Reyes AJ. Cardiovascular drugs and serum uric acid. Cardiovasc Drugs Ther. 2003;17(5–6):397–414 21. Munakata M, Imai Y, Hashimoto J, et al. The influence of antihypertensive agents on circadian rhythms of blood pressure and heart rate in patients with essential hypertension. Tohoku J Exp Med. 1992;166(2):217–27 22. Verdecchia P, Schillaci G, Borgioni C, et al. Adverse prognostic value of a blunted circadian rhythm of heart rate in essential hypertension. J Hypertens 1998;16:1335–43. 23. De Rosa ML, Cardace P, Rossi M, Baiano A, de Cristofaro A. Comparative effects of chronic ACE inhibition and AT1 receptor blocked losartan on cardiac hypertrophy and renal function in hypertensive patients. J Hum Hypertens. 2002;16(2):133–40. 24. Leonetti G. Comparison of metabolic and hemodynamic effects of hydrochlorothiazide in monotherapy and in association with lisinopril. An Italian multicenter study. Minerva Cardioangiol. 1995;43(9):389–98
Content is licensed under a Creative Commons Attribution 4.0 International License.
Share the article on social networks
Recommend the article to your colleagues