Structure FunctionsCausesSymptomsTreatmentDrugs
Cartilage tissue is present in many organs, but it is subjected to the greatest stress in the joints. Cartilage covers the vulnerable areas of the bones in the joints and provides shock absorption, as well as resistance to stress, thanks to which we do not even think about what tests the joints face in the life of an ordinary person, not to mention the categories of people who subject their bodies to excessive stress. Unfortunately, cartilage can deteriorate over time for various reasons, leading to limited joint movement, pain and discomfort. Therefore, it is so important to take the necessary measures in time to restore the cartilage tissue of the joints.
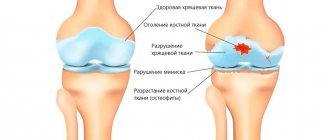
Structure of cartilage tissue
Cartilage is a type of connective tissue, and there are three types of it in the body:
- Hyaline (vitreous) - has a bluish color, with a high content of fine collagen fibers, covers the articular surfaces of bones;
- Elastic (mesh) - characterized by increased elasticity and flexibility, it is dominated by elastin fibers, it forms small bronchi and auricles;
- Fibrous - connects tendons and ligaments to the hyaline cartilage of the articular surface.
Like any tissue, cartilage consists of cells and intercellular substance (matrix), the proportion of the latter significantly predominates in it. The matrix contains a lot of water, which does not compress or stretch, but circulates freely in the intercellular space. It is water that provides high elasticity to cartilage tissue, distributing loads and cushioning. Another important component of the matrix is protein fibers: collagen and elastic. The hyaline cartilage of articular surfaces is dominated by collagen, which provides high strength. Its large molecules, twisted into a triple helix, are resistant to any deformation and quickly return to their original state. The matrix also contains glycosaminoglycans, proteoglycans, and hyaluronic acid, which retain water and participate in metabolic processes.
Cartilage cells - chondrocytes and their young forms, chondroblasts - play an equally important role: they synthesize all the components of the matrix and joint fluid. There are very few chondrocytes, only 1-5%, but they are responsible for the renewal and restoration of cartilage.
One of the main differences between cartilage tissue is the absence of blood vessels. As a consequence, cartilage must receive nutrition in an alternative way. Synovial, or joint, fluid reduces friction between articular surfaces and provides nutrition to cartilage tissue. Therefore, the delivery of nutrients and the removal of breakdown products is ensured by synovial fluid.
The effect of non-steroidal anti-inflammatory drugs on the metabolism of articular cartilage
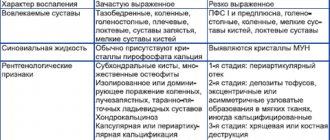
OA ranks first in prevalence among other rheumatic diseases and clearly correlates with age [1]. Radiological symptoms of this disease occur in 50% of the European population over 65 years of age, clinical symptoms in 12.5%, and among people over 80 years of age they are detected in almost all patients [2]. OA is characterized by a lack of correlation between clinical symptoms, joint radiography, magnetic resonance and computed tomography, ultrasound symptoms and the macroscopic picture obtained during arthroscopy. It is believed that treatment and secondary prevention of OA should be carried out only in individuals with a clinical picture of the disease, namely pain and dysfunction of the affected joints. This disease primarily affects the “load-bearing” joints, which significantly worsens the quality of life of patients and is a serious socio-economic problem. The most significant clinical manifestations of OA are joint pain and limited mobility. Joint pain is the most striking symptom of this disease, and the dynamics of its intensity and duration are the most important indicator of the effectiveness of the therapy. The pain is mechanical in nature, intensifying with physical activity or at the end of the day. But pain in this disease can also be of the inflammatory type, which is usually observed with the development of secondary arthritis (synovitis). The reasons for its development are extremely diverse and are due to an interest in the pathological process of bone (periostitis, subchondral fractures, increased intraosseous pressure), synovial membrane (inflammation, irritation of sensitive nerve endings by osteophytes) and periarticular tissues (muscle spasm, joint instability). The cause of pain may be other factors, for example, a decrease in the resistance of the subchondral bone to load, uneven distribution of load on various parts of the joints, degenerative changes in the periarticular tissues, and circulatory disorders of the joints. Treatment of OA includes a large range of non-drug and drug methods. The basis of pharmacotherapy for OA is non-opioid and opioid analgesics (paracetamol, tramadol), systemic non-steroidal anti-inflammatory drugs (NSAIDs), local therapy (NSAIDs, capsaicin, various patches with analgesic and anti-inflammatory activity, dimethyl sulfoxide applications). An important component of the OA treatment program are symptomatic slow-acting medications and, first of all, glucosamine sulfate or hydrochloride and chondroitin sulfate (although opinions about their effectiveness are controversial), as well as intra-articular injections of prolonged crystalline glucocorticoids and hyaluronic acid preparations. There is a large arsenal of alternative treatment methods, but their evidence base leaves much to be desired. What is the rationale for prescribing NSAIDs for OA? Although OA is considered as the main degenerative disease of the joints, convincing data have recently appeared that allow it to be considered a disease in the pathogenesis of which an important, and possibly decisive, role belongs to persistent inflammation in the tissues of the joint with the development of recurrent (secondary) synovitis, chondritis, osteitis and periarthritis. Persistent inflammation in the joint tissues contributes to the progression of morphological changes, incl. structural changes in hyaline cartilage with its degeneration and volume reduction [3]. Interestingly, at the European Congress of Rheumatology in 2012 in Berlin (Germany), the advisability of prescribing low doses of prednisolone, methotrexate and the use of monoclonal antibodies to tumor necrosis factor-α (TNF-α) for this disease was discussed. What underlies inflammation in OA? Currently, strong evidence has been obtained confirming the influence of pro-inflammatory mediators on the metabolism of hyaline cartilage cells and the progression of OA. Among the mediators responsible for its progression, interleukin-1β (IL-1β) is of key importance, which is expressed in OA-affected cartilage and stimulates the production of matrix metalloproteinases (MMPs) [4]. It is known that the hyperproduction of MMPs by chondrocytes and other cells, including collagenases (MMP-1, -8, -13), aggrecanases (ADAM-TS4 and -TS5), stromelysin-1 (MMP) plays a decisive role in the progression of pathological changes in primary OA. -3) and gelatinases (MMP-2, -9) [5]. IL-1β inhibits the expression of MMPs inhibitors, the synthesis of collagen and proteoglycans, promotes the synthesis of plasminogen activator, and at the same time stimulates the synthesis and release of certain eicosanoids, including prostaglandins (PGE) and leukotrienes. It determines the level of the catabolic process in OA and stimulates the production of other proinflammatory cytokines (IL-6, -8). The leading role of this cytokine in the development of inflammation in joint tissues is confirmed by the fact that injections of IL-Ra contribute to the resolution of clinical manifestations of OA [6]. Other inflammatory mediators are also involved in the development of OA, in particular TNF-α (TNF-α), IL-17, IL-18, oncostatin M (OSM), and leukemia inhibitory factor (LIF) [4]. The content of TNF-α is significantly lower in OA than in rheumatoid arthritis, but significantly higher than in healthy individuals. OSM, a member of the IL-6 family, plays a role in inflammation. The experiment showed that it promotes the release of proteoglycans, stromelysin-1 and collagen from cartilage tissue. OSM enhances the activity of other proinflammatory mediators, including IL-6. In turn, IL-6 induces the synthesis of tissue inhibitor MMPs. IL-18 is detected in biopsies of hyaline cartilage and synovium obtained from patients with OA. Other inflammatory mediators are also involved in the pathogenesis of OA. Osteoarthritic cartilage expresses high levels of nitric oxide (NO), which are detected in serum and synovial fluid. In vitro, strong evidence has been obtained that this mediator and its oxidized metabolites have anti-inflammatory and catabolic effects [7]. NO inhibits the synthesis of cartilage matrix macromolecules and reduces the expression of IL-1Ra by chondrocytes, participates in the synthesis of PGE2, promotes apoptosis of chondrocytes, and reduces the intensity of the anabolic process. On the other hand, PGE2 promotes joint tissue damage, potentiating other inflammatory mediators, directly affecting cartilage remodeling, MMPs production, osteoclastic bone resorption and angiogenesis [8]. All of the above justifies the prescription of anti-inflammatory therapy for OA. It seems that the positive effect of slow-acting symptom-modifying drugs, previously called chondroprotectors, is primarily associated with the suppression of persistent inflammation in the tissues of the joint and, first of all, in the hyaline cartilage. In the treatment of OA, the main symptom-modifying drugs with rapid action are simple analgesics and NSAIDs. The latter occupy a leading position, actively suppress pain and normalize the function of the affected joints (Table 1). They are indicated in the presence of secondary (reactive) arthritis or periarthritis, for example, in crow's foot syndrome, as well as in severe pain that cannot be relieved with simple or opioid analgesics. The biological activity of NSAIDs is due to mechanisms of different pathogenesis, many of which are not associated with PGE inhibition. Thus, they suppress the function of neutrophils and the interaction of leukocytes with the vascular endothelium, inhibit the activation of NF-kB (transcription factor), which is a regulator of the synthesis of proinflammatory cytokines, suppress the proliferation of some cells and induce their apoptosis, and inhibit the activity of MMPs [9]. NSAIDs are characterized by different mechanisms of action on hyaline cartilage [10, 11], but in the overwhelming majority they inhibit its metabolism. Thus, salicylates, ibuprofen and naproxen inhibit the synthesis of the main components of the cartilage matrix, including the synthesis of proteoglycans, glycosaminoglycans (GAGs) and hyaluronate, and also increase their release. Indomethacin inhibits glycosyltransferase, which is involved in the synthesis of polysaccharide chains of proteoglycans, and also disrupts the rate of incorporation of sulfates into them. Naproxen significantly reduces the content of proteoglycans and affects the activity of metalloproteinases in articular cartilage, as is the case with nimesulide. The negative effect of NSAIDs on cartilage is also due to disruption of oxidative phosphorylation in mitochondria, activation of cAMP-dependent kinase A, and disruption of protein-protein interactions at the level of the cell membrane. The vast majority of NSAIDs inhibit NO-induced apoptosis, independently of the inhibition of COX2 and PGE2 production. It should be emphasized that the data presented above on the effect of NSAIDs on the metabolism of articular cartilage were obtained mainly in an experiment on a tissue culture model of articular cartilage, and this should be taken into account when assessing the results obtained. The effect of NSAIDs on metabolic processes in chondrocytes in each specific case is related to their metabolic activity, the tested dose of the study drug, the age of the donor, the stage of OA and the degree of cartilage damage. Interesting data was presented by DA Kalbhen [12]. The author, using histological methods, X-ray and stereoelectron microscopy, as well as biochemical tests, showed that salicylates, indomethacin, phenylbutazone, naproxen, ibuprofen, dexamethasone, administered intra-articularly, cause degenerative changes in articular cartilage and subchondral bone in rats and chickens. A large number of experimental and clinical observations led to the conclusion that NSAIDs in most cases inhibit the metabolic activity of chondroblasts and chondrocytes, reduce the synthesis of proteoglycans, type II collagen and hyaluronic acid, promote premature death of chondrocytes, increase cartilage degeneration and lead to the progression of OA. The presented data gave rise to the experts of the European Group for the Respect and of Ethics and Excellence in Science (GREES) to conclude that there is not yet sufficiently convincing data indicating a beneficial effect on cartilage tissue of widely used NSAIDs (diclofenac, naproxen) and selective inhibitors cyclooxygenase-2 (COX-2) [13]. According to the experts of this group, the “ideal” NSAID should not adversely affect healthy contralateral cartilage and at the same time should stimulate the synthesis of cartilage tissue, slow down cartilage resorption and inhibit the synthesis of catabolic cytokines. Based on their effect on the metabolism of hyaline cartilage, NSAIDs can be classified into drugs that have chondronegative, chondroneutral and chondroprotective effects. Indomethacin, piroxicam, naproxen and some other traditional NSAIDs have a chondronegative effect on cartilage, ibuprofen, diclofenac have a chondroneutral effect, and aceclofenac, ketoprofen and meloxicam have a chondroprotective effect. Aceclofenac (Aertal) has established itself as a drug with high anti-inflammatory activity and good tolerability. It is a derivative of phenylacetylic acid (2-[2,6 - dichlorophenyl) amino] phenylacetoacetic acid) and is structurally similar to diclofenac. The drug does not have cumulative activity, and its pharmacokinetics does not depend on the age of the patients, which is important in the treatment of patients with OA. The anti-inflammatory effect of aceclofenac, like other NSAIDs, is realized by suppressing the synthesis of inflammatory mediators and primarily PGE2. It has been shown that in OA patients with manifestations of secondary arthritis, aceclofenac, compared to diclofenac, reduces the level of PGE2 in the synovial fluid to a greater extent, selectively suppressing the expression of COX-2. Its therapeutic effect is also associated with the inhibition of pro-inflammatory cytokines (IL-1, TNF-a) and an increase in the level of synthesis of IL-1Ra (IL-1 receptor antagonist), a decrease in the expression of adhesion molecules, the antioxidant properties of aceclofenac, which is realized by influencing free radicals, suppressing NO production in chondrocytes of healthy and OA patients, inhibition of synoviocyte proliferation [14–16]. As is known, in OA there is a dissonance between the processes of synthesis and degradation of cartilage, which is one of the characteristic manifestations of its pathogenesis. When studying samples of cartilage tissue obtained during hip replacement from patients with OA and individuals without this pathology, the effect of diclofenac and naproxen on the synthesis of GAGs was assessed. In OA, basal GAG synthesis was lower than in normal tissue samples, and the degree of decrease in their production correlated with the severity of the pathological process. Aceclofenac (0.4–10 μg/ml) significantly increased the production of GAGs in cartilage tissue affected by OA, while diclofenac in the same concentration range did not cause any effect, and naproxen significantly inhibited the synthesis of GAGs in the cartilage tissue of patients with OA (Fig. . 1). A feature of the pharmacological activity of aceclofenac and its main metabolite, 4′-hydroxyaceclofenac, is primarily the suppression of COX-2 and, to a lesser extent, COX-1 [17]. Its other metabolite, diclofenac, inhibits both COX-1 and COX-2. This explains not only its pronounced anti-inflammatory potential, but also its high safety. When taking aceclofenac, the ratio of COX-2 to COX-1 is higher than when taking standard NSAIDs, such as piroxicam, indomethacin, tenoxicam and ketoprofen. Predominant inhibition of COX-2 has been shown, in particular, in cultures of normal and OA-affected chondrocytes when incubated with aceclofenac [18]. The rationality of using aceclofenac in patients with OA is explained not only by its anti-inflammatory and analgesic properties, but also by the peculiarities of its influence on the metabolism of the main substance of hyaline cartilage (Table 2). Aceclofenac stimulates chondrocytes to produce the intercellular substance of cartilage - proteoglycans, GAGs and collagen, necessary for the full function of cartilage. It also stimulates GAG synthesis in cartilage obtained from OA patients, while diclofenac and naproxen do not have such properties. In addition, it inhibits the premature death of chondrocytes. In the experiment, aceclofenac demonstrates chondroprotective properties, suppressing IL-1-mediated production of MMPs, suppresses the expression of IL-6 by chondrocytes. It inhibits procollagenase/proMMP-1 and prostromelysin/proMMP-3 through its main metabolite 4-hydroxyaceclofenac. In terms of its anti-inflammatory activity, aceclofenac for major inflammatory diseases of the joints and spine is equivalent to diclofenac, piroxicam, indomethacin and tenoxicam. Its symptom-modifying properties have been studied in several double-blind controlled studies. The effectiveness of aceclofenac in suppressing the main manifestations of OA, namely reducing the intensity of joint pain and normalizing the functional ability of the affected joints, is significantly higher compared to placebo and is equivalent to the effectiveness of diclofenac, piroxicam and naproxen. In a comparative assessment of aceclofenac and diclofenac in 397 patients with gonarthrosis, significant positive dynamics were observed in 2 groups after 12 weeks. from the start of treatment. The unidirectionality of changes concerned not only the intensity of pain or joint function, but also the global activity of the disease [19]. But still, joint pain, as assessed by the patient, decreased more significantly during treatment with aceclofenac than during treatment with diclofenac (a decrease of 71 and 59%, respectively). The symptomatic effect of aceclofenac in OA was also compared with the effect of other NSAIDs. In a multicenter, double-blind, randomized study, the effectiveness of aceclofenac (200 mg/day) and naproxen (1000 mg/day) was studied in 374 patients [20]. In both groups by the end of 12 weeks. There was a significant decrease in the intensity of pain at rest, pain during movement or palpation of the joint, as well as resolution of exudative phenomena in the knee joints and improvement in their function. Analysis of the dynamics of the main indicators of the pathological process did not reveal any significant differences between the groups of patients, although faster positive dynamics were observed in patients who were treated with aceclofenac. In another study, aceclofenac was compared with piroxicam in patients with gonarthrosis [21]. According to the total assessment, the effectiveness of these 2 drugs was almost the same, but aceclofenac demonstrated a significant reduction in pain and normalization of knee joint function after 2 weeks. from the start of therapy, then piroxicam - only by the end of the 1st month. The rationality of using aceclofenac in the treatment of OA is determined not only by its positive effect on the metabolism of cartilage tissue and its pronounced anti-inflammatory effect, but also by its good tolerability. This seems especially important, given that OA affects mainly elderly and senile people, who tolerate NSAIDs worse than young people. A large number of comorbid states in this category of patients, as well as the probability of polypragmasis and the rationality of the combination of individual drugs that patients regularly use, are also important. The results of the large 12-month-old prospective open multicenter study of SAMM (Safety Assessment of Marketeed Medicines), which evaluated the safety of Azeclofenac and diclofenac in patients with rheumatic diseases [22]. This study is based on the study of 10,142 patients, and the prevailing majority (91.4%) had an OA. Patients of the 1st group (7890 people) took 200 mg/day of Azeclofenac, the 2nd group (2252 people)-150 mg/day of Diclofenac. The average duration of medication in both groups was approximately the same and amounted to 168.1 days for Aertal, for Diclofenac - 170 days. The total number of unwanted phenomena (nya) was reliably lower in patients taking Azeclofenac, compared with patients who were treated with diclofenac (p <0.001), which equally applies to patients who stopped taking the drug due to intolerance. Patients of the 1st group had fewer gastrointestinal reactions than in patients of the 2nd group, and the frequency of nausea, abdominal pain and diarrhea that led to the cessation of treatment was 46, 65 and 41% lower in the Azeclofenac group than in the group of Diclofenac. Meta -analysis of the results of 13 double blind randomized studies of Azeclofenac security, in which 3574 patients with OA, rheumatoid arthritis or ankylosing spondylitis, demonstrated the best safety profile of this drug compared to those of classic NSAIDs, including diclofenacin, steaken, pyroxycs and tenoxics [23 ]. In the treatment of Azeclofenac Nye, they met 1.38 times less often than that of a group of patients treated with traditional NSAIDs (p <0.001), while the abolition of therapy due to the toxicity of the Azeclofenac was also significantly less often than in patients of the comparison group. A multi -cententer study on the analysis of bleeding from the stomach and duodenum deserves attention, including And when taking NSAIDs [24]. As a result of the study, it turned out that 38% of all cases of bleeding from the upper digestive tract in people over 18 were associated with the reception of NSAIDs, which is 152 cases per 1 million population per year. The highest risk of bleeding was when taking ketorolac - 24.7. When taking the rofecoxib, it was 7.2, meloxicam - 5.7, desketoprofen - 4.9, nimesulide - 3.2, celloxiba - 0.3 and Azeclofenac - 1.4 (95% CI 0.6, 3.3) . The authors did not confirm that the selective TsOS-2 NSAIDs have a lower risk of bleeding than traditional drugs, and noted the low risk of this complication in individuals taking aceklofenac. Thus, Azeclofenac (Airtal) is NSAID, which in its anti -inflammatory and analgesic activity is not inferior to standard drugs of this class. Its use is appropriate for OA, due to the ability to stimulate cartilage cells to the production of full -fledged proteoglycans, GAG, hyaluronic acid and inhibit the apoptosis of chondrocytes, which is especially important in the light of modern ideas about OA as a disease that develops as a result of premature death of chondrocytes at the very early stages of its development. The predominant influence of Azeclofenac on the COO-2 provides its good tolerance and high safety. The positive properties of this drug increase the level of compliance with treatment compared to standard NSAIDs.
References 1. Perrot S., Menkes CJ Nonpharmacological approaches to pain in osteoarthritis. Available options // Drugs. 1996. Vol. 52. R. 21–26. 2. Sangha O. Epidemiology of rheumatic disease // Rheumatology. 2000. 39 (Suppl. 2). R. 3–12. 3. Van den Berg WB Pathophysiology of osteoarthritis // Joint Bone Spine. 2000. Vol. 67. R. 555–556. 4. Martel-Pelletier J., Pelletier J.-P. Inflammatory factors are involved in osteoarthritis. In: Osteoarthritis, Inflammation and Degradation: A Continuum. iOS Press. 2007. R. 3–13. 5. HenrotinY., Reginster T. In vitro difference among nonsteroidal antiinflammatory drugs in their activities related to osteoarthritis pathophysiology // Osteoarthritis Cartilage. 1999. Vol. 7. R. 355–357. 6. Huskinsson EC, Berry P., Gishen P. Effects of antiinflammatory drugs on the progression of osteoarthritis of the knee. J Rheumatol. 1995. Vol. 22. R. 1941–1946. 7. Abramson SB Osteoarthritis and nitric oxide // Osteoarthritis and Cartilage. 2008. Vol. 16 (Suppl 2). R. 15–20. 8. Herrero-Beaumont G., Rovati LC Use of crystalline sulfate in osteoarthritis // Future Rheumatology. 2006. Vol. 14). R. 397–414. 9. Nasonova V.A., Nasonov E.L. Rational pharmacotherapy of rheumatic diseases. M.: Litterra, 2003. 10. Ding C. Do NSAIDs affect the progression of osteoarthritis? //Inflammation. 2002. Vol. 26. R. 139–142. 11. Mastbergen SC, Biijlsma JW, Lefeber FP Selective COX-2 inhibition is beneficial to human early and late age osteoarthritis cartilage: a human in vitro study // Osteoarthritis Cartilage. 2005. Vol. 13. R. 519–526. 12. Kalbhen DA The influence of NSAIDs on the morphology of articular cartilage // Scand J Rheumatol. 1988. Vol. 77 (Suppl). R. 13–22. 13. Recommendations for the registration of drug used in the treatment of osteoarthritis // Ann Rheum Dis. 1996. Vol. 55. R. 552–557. 14. Aceclofenac. Almirall Prodespharma SA Barcelona, 2003. 120 p. 15. Grau M., Guash J., Montero Jl et al. Pharmacology of the potent new non-steroidal inflammatory agent aceclofenac // Arzneimittelforschung. 1991. Vol. 41. R. 1265–1276. 16. Murherjee P., Rachita C., Aisen PS, Pasinetti GM Non-steroidal anti-inflammatory drugs protect against chondrocyte apoptotic death // Clin Exp Rheumatol. 2001. Vol. 19 (Suppl. 22). R. 7–11. 17. Henrotin Y, De Leval H, Mathy-Hartet M et al. In vitro effects of aceclofenac and its metabolites on the production by chondrocytes of inflammatory mediators // Inflamm Res. 2001. Vol. 50. R. 391–399. 18. Blanco FJ, Maneiro E, de Toro FJ et al. Effect of NSAIDs on synthesis of IL-1 receptor by human articular chondrocytes // Lab Invest Rheumatol. 2000. THO 17. 19. Ward DE, Veys EM, Bowdler JM, Roma J. Comparison of aceclofenac with diclofenac in the treatment of osteoarthris // Clin Rheumatol. 1995. Vol. 14. R. 656–662. 20. Korsanoff D., Frerick H., Bowdler J., Montull E. Aceclofenac is a well-tolerated alternative to naproxen in the treatment of osteoarthritis. Clin Rheumatol. 1997. Vol. 16. R. 32–38. 21. Perez Busquer M., Calero E., Rodriguez M. et al. Comparison of aceclofenac with рiroхicam in the treatment of osteoarthritis // Clin Rheumatol. 1997. Vol. 16. R. 154–159. 22. Huskisson E., Irani M., Murray F. A large prospective open-label, multicentre SAMM study, comparing the safety of aceclofenac in patients with rheumatic disease. Eur J Rheumatol Inflamm. 2000. Vol. 7. R. 1–7. 23. Peris F., Bird HA, Serni U. et al. Treatment compliance and safety of aceclofenac versus NSAIDs in patients with common arthritis disorders: a meta-analysis // Eur J Rheumatol Inflamm. 1996. Vol. 16. R. 37–45. 24. Laporte J.-R., Ibanez L., Vidal H. et al. Upper gastrointestinal bleedng accociated with the use of NSAIDs. Newer versus older agents // Drug Safety. 2004. Vol. 27 (6). R. 411–420.
Causes of destruction of cartilage tissue
The cartilage in the joints is subjected to significant stress, and in certain circumstances it cannot withstand it: it begins to collapse. Especially if changes occur in the body that reduce its regenerative abilities. In this case, osteoarthritis develops - a disease caused by degenerative changes or destruction of the cartilage and bone tissue of the joints. Previously, another name for this condition was used - osteoarthritis, emphasizing the age-related nature of the changes. But, since inflammatory processes inevitably develop against the background of destruction of osteochondral structures, and it is they that cause characteristic symptoms, the term “osteoarthritis” is now predominantly used, and according to the international classification of diseases (ICD-10), both of these diagnoses are considered synonymous. In relation to dystrophic changes associated with the destruction of cartilage and bone tissue of the spine, it is customary to use the term “osteochondrosis”.
The causes of destruction of cartilage tissue are varied: