Signs of gout
Gout pain is the main symptom, usually pain in the joints of the fingers, feet, elbows, knees and others. This is precisely where the complexity lies, because these are symptoms of numerous rheumatological diseases. This circumstance explains the difficulty of diagnosis. To determine the diagnosis, doctors rely on the external manifestations of the disease, as well as on data from laboratory and instrumental diagnostic methods. Diagnosis and its complexity largely depend on the severity of the clinical picture, as well as the reasons that provoked the development of the disease. Examination, history taking, instrumental and laboratory diagnostic methods will reveal:
- hyperuricemia - increased levels of uric acid, and this is the main reason leading to the disease and provoking symptoms;
- accumulation of salt crystals (urates), including in the tissues surrounding the joints;
- an inflammatory reaction that causes severe pain.
If the disease persists for a long time, the kidneys are also affected, so assessing their functioning and early detection of failures is one of the mandatory points in the examination.
Complications without proper treatment
Lack of treatment in the early stages almost always leads to complications. They can appear after years and even with an asymptomatic form of the disease.
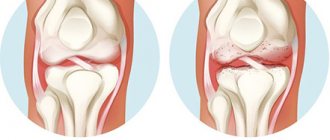
Gouty insoluble nodes (tophi) are deposited in other joints or periarticular tissues, and the inflammatory process begins. Chronic gouty arthritis develops, in which articular cartilage and ligaments are damaged, joints are destroyed, and bones are deformed. The person loses the ability to move normally, the pain becomes constant.
Urate salts can be deposited in the kidneys, leading to urolithiasis , nephropathy and gouty nephritis. Over time, kidney failure is possible. The kidneys cannot cope with their work, which becomes the cause of persistent arterial hypertension.
Beginning of the examination: survey
Joint pain and poor health are symptoms that require consultation with a rheumatologist. The signs that are characteristic of the disease are not specific, so a complex and lengthy diagnosis is needed. And an important role in this matter is to clarify complaints and anamnesis.
What worries the patient, when the first symptoms appeared, what preceded them and, most importantly, how they developed. Have you had joint pain in the past: how long ago, which joints were affected. Taking into account the hereditary factor, it is necessary to determine the family history of the disease, as well as the presence of bad habits: smoking, alcohol abuse, etc. It is important to find out how much water a person drinks per day, whether he has been taking medications for a long time and which ones, as well as kidney disease, which may affect their filtering abilities.
Development of the clinical picture
Anamnesis and examination allow, in some cases, to make a preliminary diagnosis, even before laboratory and instrumental examination methods are carried out. During an external examination, the doctor assesses the presence of an inflammatory process in the joints and surrounding tissues, the mobility of the joint, and the amplitude of its movements. Regular examinations by a doctor are necessary to assess the clinical picture, its development, to make a diagnosis and, of course, prescribe treatment for gout.
Instrumental examination methods
Some types of hardware examination will help to determine the cause of pain in the joints and tissues surrounding it. The appearance of symptoms is a reason for ultrasound, CT and radiography. It is worth remembering that in the early stages of the development of the disease, these examination methods are ineffective, since destructive processes in the tissues are not expressed. Instrumental examination methods will help identify rheumatological diseases. When prescribing instrumental diagnostics, the following nuances are taken into account:
- Ultrasound is most informative on days 3-4 after an acute attack. The study will reveal destructive changes in the joint, swelling and hardening of soft tissues. But later ultrasound is ineffective.
- CT scan will help identify tophi. This examination method is most informative in the later stages of the disease, when salt crystals form in the periarticular tissues. In the early stages of gout, CT can determine the compaction of periarticular tissues.
- X-rays in the early stages of the disease are performed to exclude other rheumatological diseases. If a diagnosis is made, it is carried out only in the chronic course of the disease to assess structural changes and the condition of the joints.
- Scintigraphy is a visualization method. Radioactive isotopes are introduced into the blood, which makes it possible to accurately determine the places where salt deposition occurred. This examination method detects the disease even in the early stages, when the formation of urates is just beginning.
What tests are needed?
Laboratory tests are carried out even in the early stages. Moreover, it is possible to calculate not only the disease in its early stages, but also predisposing factors for its development. With the help of laboratory diagnostics, a so-called differential diagnosis is carried out, that is, the difference between one disease and another. A general blood test
is a mandatory examination, which is most informative during acute attacks of the disease.
A biochemical blood test
will show an increased concentration of uric acid.
The same examination method will reveal an increased concentration of C-reactive protein, calcium, as well as an increased concentration of lipoproteins, lipids, etc. A general urine test
is mandatory, although it is carried out to assess kidney function. The amount of uric acid that is excreted by the kidneys is assessed. There is an interesting pattern here: during pathological processes in the kidneys, the volume of uric acid that is excreted in the urine is usually minimal. Based on the data obtained, a diagnosis is made, treatment tactics are developed: diet, medications for gout, physiotherapeutic treatment methods, etc. Only a doctor can treat gout, taking into account all sorts of nuances, the clinical picture and, of course, the reasons for its formation. In the following materials we will continue the conversation about the treatment of the disease.
Text: Yulia Lapushkina.
Cardiovascular risk in patients with gout and possible ways to reduce it
Gout is a systemic disease in which monosodium urate crystals are deposited in various tissues and in individuals with hyperuricemia, inflammation develops due to environmental and/or genetic factors [1]. The prevalence of gouty arthritis in the population is quite high and amounts to 5–28 per 1000 men and 1–6 per 1000 women, and the number of new cases per year is 1–3 per 1000 men and 0.2 per 1000 women [2].
Over the past decades, the incidence of gout has increased several times [3]. Despite the detailed description of gout already in the last millennium, this disease remains one of the late diagnosed processes. According to the Clinic of Therapy and Occupational Diseases named after. E. M. Tareev First Moscow State Medical University named after. I.M. Sechenov, of 128 patients with typical gout, in more than half the diagnosis was established extremely late, already with advanced organ damage [4].
Interest in the problem of gout is due not only to the increase in its incidence, but also to the discovered relationships between gout, hyperuricemia and cardiovascular risk [5]. Hyperuricemia plays an important role in the development of arterial hypertension (AH), diabetes mellitus and is an independent predictor of cardiovascular diseases [6].
The pathogenetic mechanisms of increased cardiovascular morbidity and mortality in individuals with hyperuricemia and gout are diverse. Chronic inflammation is one of the factors that triggers and maintains the formation of atherosclerotic plaque in patients with hyperuricemia and gout [7]. Oxidative stress is another important factor contributing to endothelial dysfunction and damage to the vascular wall, underlying the development of heart failure (HF), hypertension, and coronary heart disease (CHD) in patients with rheumatic diseases [8].
According to our data, which coincides with literature data [9], patients with gout belong to the group of patients with a high risk of fatal cardiovascular complications, which is due to existing endothelial dysfunction (increased antithrombogenic activity of the vascular wall, damage to the vascular wall, increased arterial stiffness). Traditional cardiovascular risk factors are more common in patients with gout than in the general population. In our study, 24-hour blood pressure (BP) monitoring revealed hypertension in 91% of patients; the majority of patients with gout (73.7%) had a 24-hour profile with an insufficient degree of nocturnal BP reduction (non-dipper) [10]. In such patients, arterial stiffness and C-reactive protein (CRP) levels are significantly increased (p < 0.05) [11].
In recent years, researchers have also found a relationship between the presence of aortic stenosis and gout, but whether gout is a risk factor or a marker of heart valve damage has yet to be studied [12]. But there is no doubt that damage to the cardiovascular system in gout, along with arthritis, is one of the main clinical manifestations of gout, determining the severity and prognosis of the disease [13]. Cardiovascular morbidity and mortality exceed mortality from renal damage in patients with gout [14].
As an illustration, we present a clinical case of a patient with gout with the development of a fatal cardiovascular complication.
Patient K., 62 years old, resident of the Saratov region, worked as a driver for 38 years. I suffered from gout for 13 years. The disease began in a typical manner, with the onset of acute attacks of arthritis in the joints of the feet, including the first metatarsophalangeal joints and tarsal joints, repeated 3–4 times a year. The attacks were controlled by taking non-steroidal anti-inflammatory drugs (NSAIDs) for several days. After 3 years from the onset of the disease, he consulted a rheumatologist, was diagnosed with gout, and was prescribed allopurinol at a dose of 200 mg/day, but the patient initially demonstrated low adherence to treatment. Allopurinol was taken only for relapse of arthritis, combined with NSAIDs. The frequency of arthritis attacks has been continuously increasing; over the past 8 years there have been no inter-attack periods. From the age of 56, the most rapid growth of subcutaneous tophi was observed, which led to deformation, disfigurement of the limbs, and a pronounced limitation in the range of motion in the joints (hands, feet, knees, ankles, wrists, elbows, shoulders). Since the age of 58, he periodically noted an increase in blood pressure (up to a maximum of 170 and 100 mm Hg), but did not receive constant antihypertensive therapy. Occasionally he took enalapril 20 mg per day.
He was hospitalized in the rheumatology department of the regional clinical hospital in April 2015. On examination: height - 178 cm, body weight - 109 kg, BMI - 34.4 kg/m2.
Attention was drawn to subcutaneous formations (tophi) in the area of the ears, the dorsum of the toes, elbows, knee joints, hands, some of large sizes (Fig.); pain, swelling of the joints of the hands and feet, ankle, knee joints; restriction of movements in them. The intensity of joint pain on a visual analogue scale is 80 mm. According to laboratory tests: serum level of uric acid (UA) in the blood - 740 µmol/l (target level 360 µmol/l), creatinine - 98.4 µmol/l, CRP - 10.4 mg/l, ESR - 45 mm/ h. After relief of arthritis, therapy with allopurinol 200 mg/day was resumed. 1 week after the start of therapy, the serum level of uric acid decreased to 504.3 μmol/l. Among the antihypertensive drugs, the patient took losartan 100 mg/day, amlodipine 5 mg/day. Against this background, blood pressure was between 140 and 90 mm Hg. Art. The patient was discharged as an outpatient with recommendations to continue therapy and titrate the dose of allopurinol until the target sUA values were achieved, which the patient did not do. I received therapy irregularly.
In February 2021, for emergency reasons, he was hospitalized in the emergency cardiology department with a clinic for acute coronary syndrome. Despite the full therapy, the patient died 14 hours after admission to the hospital from acute Q-myocardial infarction localized in the posterior wall of the left ventricle.
This clinical observation is not unique, but rather typical. Middle-aged and elderly male patients with metabolic syndrome and tophi gout, without adequate hyperuricemic therapy, extremely often die from cardiovascular complications.
New European guidelines for the management of patients with gout were published in 2021 [15]. One of the reasons for revising previously existing recommendations was the emergence of new drugs. Thus, in recent decades, IL-1 inhibitors have been used for the treatment of acute attacks, along with NSAIDs and colchicine, and non-purine xanthine oxidase inhibitors (KOIs), uricosurics, and uricase preparations have been used as urate-lowering drugs.
In our country, xanthine oxidase inhibitors are represented by two classes of drugs - purine (allopurinol, oxypurinol) and non-purine (febuxostat, topiroxostat).
According to the latest recommendations, allopurinol remains the first-line drug for urate-lowering therapy with preserved renal function. If the target sUA level cannot be achieved with an adequate dose of allopurinol, the drug should be replaced with febuxostat. Febuxostat is indicated for allopurinol intolerance and is the drug of choice for chronic kidney disease [15].
Febuxostat was recently registered in the Russian Federation. The first experience of its use in our country demonstrates the possibility of its use in patients with severe disabling gout [16]. Until recently, the lack of an alternative to allopurinol left virtually no chance of achieving the desired treatment result and preventing the progression of gout, the development of complications associated with gout and hyperuricemia [17].
An important aspect of studying the effectiveness of modern drugs is the effect of anti-gout drugs on cardiovascular risk. Preliminary reports of the use of canakinumab for the prevention of cardiovascular disease in the CANTOS (Canakinumab ANti-inflammatory Thrombosis Outcomes Sudy) study, conducted to test the inflammatory theory of atherosclerosis, results from other studies suggest a beneficial effect of the drug on outcomes associated with atherosclerosis, which is of fundamental importance for patients with gout [18].
Large studies have provided evidence that effective urate-lowering therapy not only reduces the risk of developing new attacks of arthritis and improves the quality of life of patients, but also reduces the risk of cardiovascular disease, kidney damage and mortality [19].
There are conflicting data in the literature regarding the protective effect of COIs on the cardiovascular system [20]. A population-based study in the UK found that taking allopurinol for gout was associated with a 19% reduction in the risk of death [21]. There is evidence of a decrease in the severity of endothelial dysfunction when taking allopurinol in patients with high cardiovascular risk [22].
The literature discusses the possible dose-dependent effect of allopurinol on cardiovascular risk. Thus, in the work of A. Noman et al. (2010), allopurinol in high doses (600 mg/day) in patients with angina pectoris proved to be an effective anti-ischemic agent, and in patients with heart failure, the same high doses of the drug reduced mortality twofold [23]. Whereas in more recent studies there is an indication of the leveling of the positive cardiovascular effect of allopurinol at a dose exceeding 300 mg per day [24], which may be associated with an increase in oxidative stress in conditions of high concentrations of the allopurinol metabolite - oxypurinol [25].
In 2021, the work of M. Bredemeier et al. presents a systematic review and meta-analysis of randomized trials [26], the purpose of which was to analyze the incidence of major adverse cardiovascular events, mortality (overall and cardiovascular) in randomized controlled trials using COIs compared with placebo or no treatment. The analysis included 81 articles (10,684 patients, 6434 patient-years). The results showed that the use of COI did not significantly affect the risk of major adverse cardiovascular events (relative risk (RR) 0.71, 95% confidence interval (CI) 0.46–1.09) and death (RR 0.89, 95% CI 0.59–1.33), but resulted in a reduced risk of total (RR 0.60, 95% CI 0.44–0.82), including major, cardiovascular events (RR 0. 64, 95% CI 0.46–0.89) and hypertension (RR 0.54, 95% CI 0.37–0.80). This also applied to patients with a history of cardiovascular pathology (RR 0.42, 95% CI, 0.23–0.76).
Patients receiving allopurinol had a lower risk of myocardial infarction (RR 0.38, 95% CI 0.17–0.83), hypertension (RR 0.32, 95% CI 0.18–0.58), total number (0.48, 0.31–0.75) and major cardiovascular events (RR 0.56, 95% CI 0.36–0.86). However, this effect was leveled out when the dose of allopurinol was increased above 300 mg/day, which requires further research.
The cardiovascular effects of non-purine xanthine oxidase inhibitors are less studied. As a result of selective inhibition of two forms of xanthine oxidase (oxidized and reduced), when taking febuxostat, the concentration of UA in the patient’s blood serum decreases.
The effect of febuxostat on insulin resistance (IR) and the expression of high-sensitivity CRP (hs-CRP), which is important both in the treatment of gout and in reducing cardiovascular risk, has been described [27]. In a study by J. Meng et al. 42 patients with gout and 20 control subjects, matched by gender and age, were included. Fasting insulin and blood glucose levels and hs-CRP were determined. IR was assessed using the HOMA-IR index. Patients with gout had higher levels of uric acid, insulin, HOMA-III index and hs-CRP levels than the control group (p < 0.05). After 4, 12 and 24 weeks of treatment with febuxostat, the concentrations of sUA and hs-CRP decreased significantly (p < 0.05). Insulin levels and the HOMA-IR index decreased slightly after 4 weeks of therapy and decreased significantly after 12 and 24 weeks of treatment. Thus, febuxostat can effectively control serum UA levels and improve insulin sensitivity in patients with gout.
Non-purine xanthine oxidase inhibitors have antioxidant properties by reducing the number of metabolites of purine metabolism and improving endothelial function [28]. There are studies suggesting benefits of febuxostat over allopurinol in reversing endothelial dysfunction [28] and even improved scores in patients treated with febuxostat compared with allopurinol [29]. At the same time, according to the instructions for medical use of febuxostat, the use of the drug is not recommended in patients with coronary artery disease or congestive heart failure, since, according to the results of long-term large-scale studies, in the group of patients receiving febuxostat, compared with the group of patients receiving allopurinol, there was an increase number of cardiovascular system disorders [30]. There were numerically more cardiovascular events in the febuxostat group for the composite endpoint of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death. However, these differences with the allopurinol groups did not reach statistical significance and were not replicated in the CONFIRMS study [31].
Randomized clinical trials are currently ongoing to evaluate the effect of febuxostat on cardiovascular risk in patients with gout [32].
Thus, patients with gout have an increased cardiovascular risk. Adequate urate-lowering therapy can not only normalize blood uric acid levels, but also improve the life prognosis of patients with gout by reducing the risk of developing cardiovascular diseases. In the clinical observation we presented, the lack of adequate urate-lowering therapy was one of the factors in the development of an unfavorable outcome. Further research is required to clarify the mechanisms and features of the effect of various xanthine oxidase inhibitors on cardiovascular risk.
Literature
- Rheumatology. National leadership / Ed. E. L. Nasonova. M.: GEOTAR-Media, 2008. 372 p.
- Barskova V. G., Nasonova V. A. Gout: clinical course, diagnosis, treatment. Quality of life // Medicine. 2003. No. 3. P. 49–53.
- Arromdee E., Michet CJ, Crowson CS Epidemiology of gout: is the incidence rising? // J. Rheumatol. 2012; 29: R. 2403–2406.
- Mukhin N. A. Gout: faces of the disease // Modern rheumatology. 2007. No. 1. P. 5–9.
- Eliseev M. S., Denisov I. S., Markelova E. I. et al. Independent risk factors for the development of severe cardiovascular complications in men with gout: results of a 7-year prospective observation // Therapeutic archive. 2021. No. 89 (5). pp. 10–19.
- Feig DI, Kang DH, Johnson RJ Uric acid and cardiovascular risk // N. Engl. J. Med. 2008; 359(17): P. 1811–1821.
- Perez-Ruiz F., Becker MA Inflammation: a possible mechanism for a causative role of hyperuricemia/gout in cardiovascular disease // Curr. Med. Res. Opin. 2015; 31. Suppl 2: 9–14.
- Richette P., Perez-Ruiz F., Doherty M. et al. Improving cardiovascular and renal outcomes in gout: what should we target? //Nat. Rev. Rheumatol. 2014; 10. P. 654–661.
- Zhu Y., Pandya B., Choi H. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008 // Am. J. Med. 2012: 125. P. 679–687.
- Rebrov A.P., Magdeeva N.A. Use of amlodipine for arterial hypertension in patients with gout // Saratov Journal of Medical Scientific Research. 2008. No. 1 (19). pp. 72–75.
- Rebrov A.P., Magdeeva N.A. Features of endothelial dysfunction in patients with gout and its changes during therapy // Saratov Journal of Medical Scientific Research. 2008. No. 3 (21). pp. 59–62.
- Chang K., Yokose C., Tenner C. et al. Association between gout and aortic stenosis // Am. J. Med. 201 7; 130 (2): 230.e1–230.e8.
- Eliseev M. S., Denisov I. S., Markelova E. I. et al. Independent risk factors for the development of severe cardiovascular complications in men with gout: results of a 7-year prospective study // Therapeutic archive. 2021. No. 89 (5). pp. 10–19.
- Krishnan E., Baker JF, Furst DE et al. Gout and the risk of acute myocardial infarction // Arthritis Rheum. 2006 Aug; 54 (8): pp. 2688–2696.
- Eliseev M. S. Updated EULAR recommendations for the treatment of gout. Comments on some positions // Scientific and practical rheumatology. 2021. No. 55 (6). pp. 600–609.
- Eliseev M. S., Shayakhmetova R. U. Experience of using febuxostat in a patient with severe disabling gout // Modern Rheumatology. 2021. No. 11 (3). pp. 81–84.
- Eliseev M. S., Barskova V. G., Denisov I. S. Dynamics of clinical manifestations of gout in men (data from a 7-year retrospective observation) // Therapeutic archive. 2015. No. 87 (5). pp. 10–15.
- Eliseev M. S., Zhelyabina O. V., Markelova E. I. et al. Assessment of cardiovascular risk when using an interleukin 1 inhibitor in patients with severe tophi gout // Modern rheumatology. 2016. No. 10 (1). pp. 7–14.
- Goicoechea M., de Vinuesa S.G., Verdalles U. et al. Effect of Allopurinol in Chronic Kidney Disease Progression and Cardiovascular Risk // Clin. J. Am. Soc. Nephrol. 2010; 5 (8): pp. 1388–1393.
- Higgins P., Dawson J., Lees KR et al. Xanthine oxidase inhibition for the treatment of cardiovascular disease: a systematic review and meta-analysis // Cardiovasc. Ther. 2012; 30. P. 217–226.
- Dubreuil M., Zhu Y., Zhang Y. et al. Allopurinol initiation and allcause mortality in the general population // Ann. Rheum. Dis. July 2015; 74 (7). P. 1368–1372.
- Xin W., Mi S., Lin Z. Allopurinol therapy improves vascular endothelial function in subjects at risk for cardiovascular diseases: a meta-analysis of randomized controlled trials // Cardiovasc. Ther. 2016; 34. P. 441–449.
- Noman A., Ang D.S., Ogston S. et al. Effect of high-dose allopurinol on exercise in patients with chronic stable angina: a randomized, placebo controlled crossover trial // Lancet. 2010; 375 (9732). P. 2161–2167.
- Givertz MM, Anstrom KJ, Redfield MM, Deswal A. Effects of Xanthine Oxidase inhibition in Hyperuricemic heart failure patients: the Xanthine Oxidase inhibition for Hyperuricemic heart failure patients (EXACT-HF) study // Circulation. 2015; 131. R. 1763–1771.
- Stamp LK, Turner R., Khalilova IS, Zhang M. et al. Myeloperoxidase and oxidation of uric acid in gout: implications for the clinical consequences of hyperuricaemia // Rheumatology (Oxford). 2014; 53. R. 1958–1965.
- Bredemeier M., Lopes LM, Eisenreich MA et al. Xanthine oxidase inhibitors for prevention of cardiovascular events: a systematic review and meta-analysis of randomized controlled trials // BMC Cardiovasc. Discord. 2021, Feb 7; 18(1):24.
- Meng J., Li Y., Yuan X., Lu Y. Effects of febuxostat on insulin resistance and expression of high-sensitivity C-reactive protein in patients with primary gout // Rheumatol. Int. Feb 2021; 37(2). P. 299–303.
- Tsuruta Y., Kikuchi K., Tsuruta Y. et al. Febuxostat improves endothelial function in hemodialysis patients with hyperuricemia: a randomized controlled study // Hemodial. Int. 2015; 19. P. 514–520.
- Tausche AK, Christoph M., Forkmann M. et al. As compared to allopurinol, urate-lowering therapy with febuxostat has superior effects on oxidative stress and pulse wave velocity in patients with severe chronic tophaceous gout // Rheumatol Int. 2014; 34. P. 101–119.
- Schumacher HR Jr., Becker MA, Wortmann RL et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, doubleblind, parallel-group trial // Arthritis Rheum. 2008; 59 (11). P. 1540–1548.
- Becker MA, Schumacher HR, Espinoza LR et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial // Arthritis Res. 2010, 12 (2): R63.
- MacDonald TM, Ford I., Nuki G. et al. Protocol of the Febuxostat versus Allopurinol streamlined trial (FAST): a large prospective, randomized, open, blinded endpoint study comparing the cardiovascular safety of allopurinol and febuxostat in the management of symptomatic hyperuricaemia // BMJ Open. 2014; 4:e005354.
N. A. Magdeeva, Candidate of Medical Sciences I. A. Romanova, Candidate of Medical Sciences N. M. Nikitina1, Doctor of Medical Sciences, Professor
FSBEI HE SSMU im. V. I. Razumovsky Ministry of Health of the Russian Federation, Saratov
1 Contact information
Cardiovascular risk in patients with gout and possible ways to reduce it / N. A. Magdeeva, I. A. Romanova, N. M. Nikitina For citation: Attending physician No. 9/2018; Page numbers in the issue: 82-85 Tags: gouty arthritis, cardiovascular complications, risk group, hypertension
Main symptoms and treatment of gouty arthritis
Patients are bothered by severe pain in the joints: even the slightest touch to them becomes unbearable, while the joints become very swollen, the skin over them acquires a purple tint and its temperature is increased. Often the patient’s body temperature rises, chills appear, and blood tests reveal an increased level of uric acid.
An exacerbation very often occurs suddenly, often at night, and lasts on average from two to five days, sometimes going away even without treatment. However, after some time the pain may return again. The intervals between attacks become shorter, and the attacks themselves become longer.
In 10% of patients, after the first attack of the disease, remission occurs (it can last up to 10 years or more). In other patients, relapses may occur two to three times a year. With each subsequent attack, the number of joints involved increases, and relapses become more severe and prolonged.
The presence of tophi nodules on the hands, feet, as well as under the skin on the ears and on the back surface of the elbow joints is a manifestation of the chronic form of gout, since they usually appear no earlier than three or more years after the first gouty attack and their number can vary. Sometimes tophi nodules form not only under the skin, but also in the kidneys and other internal organs (this is usually detected by ultrasound, CT).
Very often, gouty arthritis of the joints is combined with diabetes mellitus, obesity and hypertension, and atherosclerosis. In 1/3 of patients with gout, urolithiasis develops, and sometimes renal failure develops. All these diseases mutually aggravate each other. But in some patients, an attack of urolithiasis is the first manifestation of gout, which is detected after examining the patient for urolithiasis.