Pharmacological properties of the drug Teraflex Advance
Chondroitin sulfate is a high molecular weight mucopolysaccharide that takes part in the construction of cartilage tissue. Reduces the activity of enzymes that destroy articular cartilage and stimulates the regeneration of the latter. In the early stages of the inflammatory process, chondroitin sulfate reduces its activity and thus slows down the degeneration of cartilage tissue. Eliminates pain, improves joint function, reduces the need for NSAIDs in patients with osteoarthritis of the knee and hip joints. Glucosamine sulfate has chondroprotective properties, reduces the deficiency of glycosamines in the body, and takes part in the biosynthesis of proteoglycans and hyaluronic acid. Having an affinity for cartilage tissue, glucosamine initiates the process of sulfur fixation during the synthesis of chondroitinsulfuric acid. Glucosamine sulfate selectively acts on articular cartilage, is a specific substrate and stimulator of the synthesis of hyaluronic acid and proteoglycans, inhibits the formation of superoxide radicals and enzymes that cause damage to cartilage tissue (collagenase and phospholipase); prevents disruption of glycosaminoglycan biosynthesis induced by NSAIDs and the destructive effect of glucocorticoids on chondrocytes. Ibuprofen has analgesic, anti-inflammatory, and antipyretic effects. The mechanism of action of ibuprofen occurs due to the selective blocking of cyclooxygenase (COX-1 and -2), the main enzyme in the metabolism of arachidonic acid, which leads to a decrease in the synthesis of prostaglandins. Reduces morning stiffness of joints, helps increase range of motion in joints and spine. Pharmacokinetics. Absorption: after a single oral dose of the drug in an average therapeutic dose, the maximum concentration of chondroitin sulfate in the blood plasma is achieved after 3-4 hours, in the synovial fluid - after 4-5 hours. Bioavailability of the drug is 13%. Excretion is carried out mainly by the kidneys within 24 hours. 90% of glucosamine taken enterally is absorbed in the intestine. Over 25% of the dose taken penetrates from the blood plasma into the cartilage tissue and synovial membranes of the joints. In the liver, part of the drug is metabolized to form urea, carbon dioxide and water. The bioavailability of glucosamine is 25% due to primary passage through the liver. The maximum concentration of glucosamine is determined in articular cartilage, liver and kidneys. About 30% of the dose taken persists for a long time in bone and muscle tissue. It is excreted primarily in urine unchanged, and partially in feces. The half-life is 68 hours. Ibuprofen, when administered orally, is almost completely absorbed from the gastrointestinal tract. Eating food at the same time slows down the rate of absorption. Metabolized in the liver (up to 90%). The half-life is 2–3 hours; 80% of the dose taken is excreted in the urine, mainly in the form of metabolites.
Treatment of pain in patients with osteoarthritis of various localizations
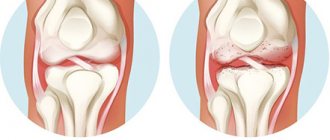
Osteoarthritis (OA) is a chronic progressive disease, a characteristic feature of which is the presence of destructive changes in articular cartilage and subchondral bone with the development of marginal osteophytes.
OA is the most common joint disease, clinical symptoms of which are generally observed in more than 10–20% of the world's population [1]. During the International Decade of Bone and Joint Diseases (2000–2010), the following diseases were identified as having the most important medical and social significance for society: osteoarthritis (osteoarthrosis), osteoporosis, low back pain, rheumatoid arthritis, traumatic injuries. In terms of its impact on health, OA ranks 4th among all diseases in women and 8th in men. Major risk factors for the disease include age, obesity, and traumatic joint injury.
Knee joints are most often affected by OA (about 10% of the population over 55 years of age), while 25% of them develop severe impairments in functional activity [2]. The risk of disability in the group of patients with gonarthrosis is comparable to the group of elderly patients suffering from cardiovascular diseases, and higher than in other diseases in these patients. The high disability of patients with OA of the knee joints is the reason that the annual incidence of arthroplasty operations among patients over 65 years of age in Europe averages 0.5–0.7 per 1000 population [3]. The high prevalence of osteoarthritis (OA) and the disability problems associated with this disease are extremely relevant for Russia. In the population among people over 15 years of age, according to a large-scale domestic study that included examination of 41,348 people, clinical manifestations of OA were identified in 6.43% [4]. The number of patients with OA in our country is about 10–12% of the population, about a third of them have some degree of disability, and therefore timely and effective treatment of OA is of great social and economic importance.
Treatment of OA is difficult for a number of reasons. The main goal of treating patients is rational pain relief and anti-inflammatory therapy, slowing the progression of the disease and maintaining the quality of life of patients. According to the modern classification of drugs used in the treatment of OA [5], they are divided into the following groups:
- Symptomatic rapid-acting drugs (non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen, opioid analgesics, corticosteroids, etc.) that affect the clinical symptoms of the disease (pain, inflammation, etc.).
- Delayed-acting modifying agents (glucosamine, diaceriin, chondroitin, hyaluronic acid, avocado and soybean unsaponifiables), the effect of which appears more slowly than symptomatic agents and lasts after the end of their use. These pharmacological agents have a chondromodifying effect, preventing the degradation of articular cartilage.
Treatment of OA begins with symptomatic therapy. The doctor should strive to provide reliable pain relief to a patient with OA, which is difficult mainly due to the elderly age of this group of patients and the presence of a large number of concomitant diseases [6, 7]. Traditionally used NSAIDs have a symptomatic effect, reducing pain and inflammation in the joints. The main disadvantage of these drugs is the presence of severe adverse reactions, especially in relation to the gastrointestinal tract and cardiovascular system; Moreover, some of them negatively affect the metabolism of articular cartilage [8, 9].
The use of drugs that can potentially modify metabolic processes in cartilage has attracted attention primarily because of their safety in the treatment of OA. They are characterized, on the one hand, by an effect comparable to NSAIDs (although more slowly developing) on pain and joint function, and on the other hand, by the ability to influence the course of the disease and its outcome, slowing down the progression of the disease. It was in the 2003 EULAR recommendations that glucosamine sulfate and chondroitin sulfate were identified as drugs with a “chondroprotective” effect. The final list of 10 recommendations of the EULAR special commission for the treatment of knee OA, based on evidence-based medicine and expert opinion, indicates the need to include symptomatic slow-acting drugs (glucosamine sulfate, chondroitin sulfate, avocado/soybean unsaponifiables, diacerein and hyaluronic acid), which have the ability to modify the structure of cartilage [5]. Numerous studies have shown that chondroitin and glucosamine have moderate to significant effects on pain and functional joint mobility in OA compared with placebo; these drugs are safe and characterized by a minimum of side effects [10–13]. The pharmacological effects of GA and cholesterol are presented in Table. 1.
According to a meta-analysis that included all available published studies on this problem, the severity of the therapeutic effect for chondroitin sulfate and glucosamine sulfate was 0.78 and 0.44, respectively [5].
Many studies have been conducted to study the effectiveness of glucosamine and chondroitin in the treatment of OA of the knee and hip joints. Research results have been summarized in reviews and meta-analyses [13–15]. In a meta-analysis by F. Richi et al. [15] showed that glucosamine has a structural-modifying (with three-year intake it slows down the degenerative process in articular cartilage) and symptomatic effect, chondroitin has a symptomatic effect (has a positive effect on the Lequesne index, visual analogue scale (VAS) indicators). However, safety during administration was excellent for both glucosamine and chondroitin.
When studying the mechanism of action of glucosamine and chondroitin sulfate, it turned out that in addition to common mechanisms, there are also differences in the effect of these substances on joint tissue. Glucosamine (GA), produced in the body as glucosamine 6-phosphate (GK6-P), is a fundamental building block required for the biosynthesis of compounds such as glycolipids, glycoproteins, glycosaminoglycans, hyaluronate and proteoglycans. Chondroitin sulfate (CS) is an important class of glycosaminoglycans required for the formation of proteoglycans found in articular cartilage [16]. The primary biological role of GA in stopping or preventing joint degeneration is directly due to its ability to act as an essential substrate for stimulating the biosynthesis of glycosaminoglycans and hyaluronic acid, necessary for the formation of proteoglycans found in the structural matrix of the joint. CS, whether absorbed intact or in the form of components, provides an additional substrate for the formation of a healthy joint matrix, since cholesterol is a component of proteoglycans (macromolecules containing many glycosaminoglycan molecules) attached to a long chain of hyaluronic acid (hyaluronate). Both GA and cholesterol are able to increase the synthesis of proteoglycans and collagen, reduce the activity of leukocyte elastase, collagenase and aggrekenase, and suppress IL-1-stimulated synthesis of prostaglandins by fibroblasts [17–19]. At the same time, there are some fundamental differences regarding, first of all, the effect on the subchondral bone and synovial membrane. It is believed that the ability to normalize bone turnover is characteristic of cholesterol, as well as the ability to mobilize fibrin, lipids and cholesterol deposits in the synovium and subchondral blood vessels, as well as reduce chondrocyte apoptosis [20]. This fact served as a prerequisite for the creation of combination drugs with the aim of potentiating the complementary effects of cholesterol and G, as well as implementing the entire spectrum of their mechanisms of action [21].
Available evidence of the symptom-modifying (reduction of pain and, accordingly, the need for anti-inflammatory drugs, improvement of the functional state of patients) and chondroprotective effects of GA sulfate and cholesterol also contributed to the creation of drugs based on their combination in order to obtain a possible overall greater effect compared to monotherapy with these drugs. According to L. Lippielo et al. (1999), the combined use of cholesterol and GA hydrochloride in an experiment increased the production of glycosaminoglycans by chondrocytes by 96.6% compared to 32% with monotherapy [22]. The use of another substance, GA hydrochloride, instead of GA sulfate in combination preparations is associated with its higher stability and bioavailability [23] (Table 2).
The drug Theraflex is also a combination of two salts - chondroitin sulfate (400 mg) and glucosamine hydrochloride (500 mg) in one capsule. There is another form of release of the drug for oral administration: Teraflex Advance, which contains chondroitin sulfate 200 mg, glucosamine sulfate 250 mg and ibuprofen 100 mg.
A preclinical study of the effect of Teraflex on cartilage structures and cells was carried out at the Department of Traumatology and Orthopedics of the Rostov State Medical University (Professor V. D. Sikilind, A. V. Alabud). Experimental studies were performed on dogs and rabbits with modeling of various fractures. After modeling intra-articular fractures, metal osteosynthesis of fractures was performed using various extraosseous and transosseous fixators with joint unloading and pharmacological protection of cartilage (structure-modifying therapy with Theraflex). The experiment studied the features and dynamics of cartilage regeneration without and with the use of drug protection during various treatment methods (Fig. 1). A total of 120 surgical interventions were performed in 6 series of experiments on chinchilla dogs and rabbits. Under the influence of drug therapy with a combination of cholesterol and GA, the number and size of chondrocytes per unit area of articular cartilage objectively increases. The most effective recovery occurred in the group of animals where fractures with a small area of damage to the articular cartilage were modeled. The greatest degenerative changes in cartilage were observed after modeling fractures with a significant area of damage to articular cartilage without additional stimulation of reparative regeneration of glycosaminoglycans.
L. I. Alekseeva et al. (Institute of Rheumatology, Russian Academy of Medical Sciences) [24], when assessing the effectiveness and tolerability of the drug Teraflex in 50 patients with significant gonarthrosis, obtained results that served as the basis for modifying the dose of Teraflex. For 4 months, patients received Theraflex (2 capsules per day for the first 3 weeks, then 1 capsule per day) and ibuprofen (400 mg 3 times a day with the possibility of subsequent dose reduction) and were observed for 2 months without treatment. A decrease in pain, stiffness and degree of functional impairment according to the WOMAC index was noted by the end of drug use in 37%, 42% and 24% of patients, respectively. At the same time, in 26 patients it was possible to reduce the dose of ibuprofen from 1200 to 800 mg per day, and in 3 patients, NSAIDs were discontinued. However, in 11 patients, when the Teraflex dose was reduced from 2 capsules/day to 1 capsule/day, an increase in joint pain was noted, so the Teraflex dose was again increased to 2 capsules per day, which allowed them to achieve an effect. 47 patients completed the full 6-month course of treatment.
Currently, the Teraflex dose is 3 capsules per day for the first 3 weeks, then 2 capsules per day for 3 months or more.
According to the Department of Clinical Geriatrics and Organization of Gerontological Care (RMAPO) [25], in postmenopausal women with an average age of 54.2 ± 7.6 years, the use of Teraflex for 6 months allowed a significant reduction in the main manifestations of OA (Table 3) with a decrease in the need in NSAIDs in 21% of patients and refusal to take NSAIDs in another 14% of patients. The authors also assessed the possibility of combining Theraflex with hormone replacement therapy for surgical menopause and showed that this combination does not reduce the effectiveness of therapy and does not affect the incidence of adverse events.
The possibility of using Teraflex in a continuous and intermittent course was studied [26]: 50 patients received Teraflex according to the usual regimen for 9 months and 50 patients received Teraflex for 3 months, then a 3-month break in treatment was taken, then again patients in this group received Teraflex. In general, by 9 months of the study, no significant differences were found between the effectiveness of the drug in both groups, although the severity of the effect was higher with continuous use of the drug (Fig. 2). In both groups, 34% of patients stopped taking NSAIDs. Ultrasound examination showed improvement (reduction in the synovium, area of the suprapatellar volvulus, size of the popliteal cyst and severity of periarticular changes) in 83% of patients who received Teraflex continuously, and in 81.2% of patients who received Teraflex intermittently.
The effect of Teraflex Advance was studied at the Institute of Rheumatology of the Russian Academy of Medical Sciences (L. I. Alekseeva et al.) in comparable groups of 20 people with gonarthrosis, whose average age was about 58 years [27]. Patients received ibuprofen (600–1200 mg/day) for 3 months in combination with Teraflex Advance 6 capsules/day (group 1), Teraflex 2 capsules daily (group 2) or ibuprofen alone 600–1200 mg/day (3rd group). The researchers noted equal effectiveness of both forms of the drug, clearly superior to the effectiveness of ibuprofen in relation to pain, stiffness and functional impairment (WOMAC index). A significant reduction in pain was noted in all three groups, but in patients receiving only ibuprofen, the reduction in pain was significantly less than in the other two groups. In the groups of patients receiving Teraflex and Teraflex Advance, stiffness scores decreased significantly after 2 months of therapy, while when taking ibuprofen alone, the reduction in stiffness did not reach statistical significance throughout the entire course of therapy. At the same time, indicators of functional impairment and the total WOMAC index when taking Theraflex Advance significantly decreased after 1 month of treatment, when taking Theraflex - after 2 months and were significantly lower than when using only ibuprofen. According to V.V. Povoroznyuk [28], taking Teraflex Advance 2 capsules 2 times a day made it possible to achieve an analgesic effect after 2 weeks of administration, and when taking the usual form of Teraflex (1 capsule 2 times a day) - after 2 months. Both forms of the drug are well tolerated. It is believed that GA and ibuprofen have a synergistic antinociceptive effect, which explains the choice of ibuprofen for combination with Theraflex. It is proposed to begin therapy for patients with OA by taking Teraflex Advance for the first 2–3 weeks to more quickly achieve an analgesic effect, followed by taking the usual form of Teraflex for another 4–6 months.
Treatment of OA, like other chronic diseases, is most effective in the early stages. According to M. S. Svetlova [29], long-term use of Teraflex in patients with OA with a disease duration of no more than 36 months (on average 11.5 months) can significantly improve the quality of life, which manifests itself after 1–2 years of therapy, which the author conducted according to the following scheme: the first month 3 capsules/day, from the 2nd to the 6th month — 2 capsules/day and repeated courses for 2 months with an interval of 1 month, 2 capsules/day. In the control group, which received only NSAIDs, the effect was maximal in the first 6 months, and then gradually lost, and after 3 years of therapy, the severity of all indicators approached the original level. After 2 and 3 years of treatment with Theraflex, all clinical indicators, with the exception of pain severity according to VAS at rest, were significantly lower than in the control group. At the same time, among patients receiving Theraflex, the need for taking NSAIDs significantly decreased, so that after 6 months 22% of patients stopped taking them, after a year - 26.4%, after 2 years - 27.5% and after 3 years - 27 .7% of patients. These data indicate that the administration of basic therapy for OA should not be delayed and should be carried out for a sufficiently long time.
There is data [30] on the effectiveness of Theraflex in patients with osteochondrosis (OC). The drug Teraflex was used in the complex treatment of 40 patients with degenerative diseases of the cervical, thoracic and lumbar spine with acute and chronic pain of varying intensity. Three patients had widespread AC with damage to several parts of the spine. The average age of the patients was 48.5 years. Teraflex was administered orally, regardless of food intake according to the standard regimen. The symptoms that patients had with damage to the cervical spine are presented in Table. 4 and for lesions of the lumbar region in table. 5. X-ray examination of all patients in the affected area revealed a decrease in the height of the intervertebral discs, subchondral sclerosis of the vertebrae, narrowing of the joint space, incongruity of the articular surfaces, and the formation of osteophytes. Controlled limitation of spinal function was observed in 100% of patients.
Treatment results were assessed by changes in symptoms and the Oswester Disability Questionnaire for back pain [31], before drug use, on the 7th, 14th and 21st days of the study and monthly until the end of the 4-month course of therapy.
The results of the study showed that a significant improvement in the condition with relief of pain and positive dynamics of neurological symptoms was observed in 75% of patients (Fig. 3), mostly young. In 20% of patients, the intensity of the pain syndrome decreased; the effect was absent in only 5% of patients. The dynamics of restoration of range of motion corresponded to a decrease in pain. The drug was well tolerated in all patients; only 2 patients had a single bowel disorder, which was not the reason for discontinuation of Teraflex therapy. No allergic manifestations were noted.
Thus, the use of the drug Teraflex in the treatment of degenerative-dystrophic diseases of the spine [30] is rational, especially in young patients, both in combination with NSAIDs and as monotherapy. In combination with NSAIDs, the analgesic effect occurred 2 times faster, and the need for therapeutic doses of NSAIDs progressively decreased.
Conclusion
- Theraflex drug is a drug that has a reliable symptom-modifying effect (reducing pain, reducing stiffness, improving motor activity) in patients with OA of large joints and the spine.
- The effectiveness of Theraflex is equivalent with continuous use for more than 6 months and with intermittent use for 3 months with a three-month break.
- Consecutive use of Teraflex Advance with a transition to Teraflex allows you to achieve a faster analgesic effect and thereby improve patient adherence to the course of treatment.
- The use of Theraflex can reduce the need for NSAIDs.
- Long-term (2–3 years) repeated courses of Theraflex can achieve a long-term symptom-modifying effect.
- There is evidence of pain relief, with positive dynamics of neurological symptoms in patients with osteochondrosis while taking Theraflex.
- Theraflex drug is well tolerated with long-term use.
Literature
- Smith MM, Ghosh P. Osteoarthritis: Current status and future directions // APLAR J Rheum. 1998; 2:27–53.
- Lawrence RC, Brummer JM, Bier F. Osteoarthritis prevalence in the population and relationship between symptoms and x-ray changes // Ann Rheum Dis. 1966; 25:1–24.
- Lawrence RC, Helmick CG, Arnett FC et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States // Arthritis Rheum. 1998; 41: 778–799.
- Benevolenskaya L. I., Brzhezovsky M. M. Epidemiology of rheumatic diseases. M.: Medicine, 1988.
- Jordan KM, Arden NK, Doherty M. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT) // Ann Rheum Dis. 2003; 62:1145–1155.
- Vertkin A.L., Alekseeva L.I., Naumov A.V. et al. Osteoarthrosis in the practice of a general practitioner // RMZh. 2008; vol. 16, no. 7: 33–37.
- Van Dijk G.V., Veenhof C., Schelleviset S. et al. Comorbidity, limitations in activities and pain in patients with osteoarthritis of the hip or knee BMC Musculoskelet // Disord. 2008; 9:95.
- Nasonov E. L., Lazebnik L. B., Belenkov Yu. N., etc. The use of non-steroidal anti-inflammatory drugs. Clinical recommendations. M., 2006. 88 p.
- Blot L., Marcelis A., Devogelaer JP, Manicourt DH Effects of diclofenac, aceclofenac and meloxicam on the metabolism of proteoglycans and hyaluronan in osteoarthritic human articular cartilage // Br J Pharmacol. 2000, 131: 1413–1421.
- Delafuente JC Glucosamine in the treatment of osteoarthritis // Rheum Dis Clin North Am. 2000; 26:1–11.
- Houpt JB, McMillan R. et al. Effect of treatment of glucosamine hydrochloride in the treatment of pain in osteoarthritis of the knee // J Rheum. 1998; 25; suppl. 52:8.
- Mazieres B., Combe B., Phan Van V. et al. Chondroitin sulfate in osteoarthritis of the knee: a prospective, double blind, placebo controlled multicenter clinical study // J Rheum. 2001; 28: 173–181.
- McAlindon TE, LaValley MP, Gulin JP, Felson DT Glucosamine and chondroitin for the treatment of osteoarthritis: a systematic quality assessment and meta-analysis // JAMA. 2000; 283:1469–1475.
- Leeb BF, Schweitzer H., Montag K., Smolen JS A meta-analysis of chondroitin sulfate in the treatment of osteoarthritis // J Rheumatol. 2000; 27:205–211.
- Richy F., Bruyere O., Ethgen O. Structural and symptomatic efficacy of glucosamine and chondroitin in knee osteoarthritis: a comprehensive meta-analysis // Arch Intern Med. 2003; 163:1514–1522.
- Povoroznyuk V.V. Glucosamine and chondroitin in the treatment of osteoarthritis: literature data and results of our own research // RMZh. 2006; vol. 14, no. 4: 1–5.
- Baici A., Bradamante P. Interaction between human leukocyte elastase and chondroitin sulfate // Chem Biol Interaction. 1984; 51:1–11.
- Lippielo L., Grande D. In vitro chondroprotection of glucosamine and chondroitin sulfate in a rabbit model of a OA and demonstration of metabolic synergy on chondrocyte in vitro // Ann Rheum Dis. 2000; 59 (Suppl. 1): 266.
- Yaron I., Shirasi R., Judovich R., Yaron M. Chondroitin sulfate inhibits prostaglandin E2 production in synovial cell cultures and reverses IL-1 inhibition of cartilage synthesis // Ann Rheum Dis. 2000; 59 (Suppl. 1): 265.
- Reveliere D., Mentz F., Merie-Beral H. et al. Protective effect of chondroitin 4,6-sulfate on apoptpsis of rabbit articular chondrocytes - preliminary results. In: Mautone G., Tajana E., Rovati S., Vacher D. editors. New appoaches in OA. Zurich: Litera Rheumatologica 24, EULAR; 1999: 15–20.
- Benevolenskaya L. I., Alekseeva L. I., Zaitseva E. M. Efficacy of the drug Teraflex in patients with osteoarthritis of the knee and hip joints (open randomized trial) // RMZh. 2005; 13(No. 8): 525–527.
- Lippielo L., Woodword J., Karpman D. et al. Beneficial effect of cartilage structure modifying agents tested in chondrocyte and rabbit instability model osteoarthrosis // Arthr Rheum. 1999; Suppl. 42:256.
- Lila A. M., Mazurov V. I., Shidlovskaya O. V., Shostak M. S. Teraflex in complex therapy of osteoarthritis of the knee joints and spine (results of a clinical study) // RMJ. 2005; 13, No. 24: 1618–1622.
- Alekseeva L. I., Benevolenskaya L. I., Zaitseva E. M. Efficacy of the drug Teraflex in patients with osteoarthritis of the knee and hip joints (open randomized trial) // RMZh. 2005; 13(No. 8): 525–527.
- Malichenko S. B., Kolesova I. R. Study of the clinical effectiveness and tolerability of the drug Teraflex (glucosamine hydrochloride - 500 mg and sodium chondroitin sulfate - 400 mg) for articular syndrome in women in physiological and surgical menopause (report). M., 2005.
- Alekseeva L. I. Results of an open comparative randomized study of the effectiveness and safety of 2 treatment regimens with Theraflex in patients with osteoarthritis of the knee joint. Clinical study // Breast cancer. 2008, vol. 16, no. 5: 316–319.
- Alekseeva L. I. An open randomized comparative study of the effectiveness and safety of the drug Teraflex Advance compared with the drugs Teraflex and ibuprofen in patients with osteoarthritis of the knee joints. Scientific report. 2008.
- Povoroznyuk V.V. The effectiveness of the drug Teraflex Advance in the treatment of pain syndrome in osteoarthritis of the knee joints // Health of Ukraine. 2007; 1–3.
- Svetlova M. S. The effect of long-term therapy with Theraflx on symptoms and quality of life in patients with early stages of gonarthrosis // Modern Rheumatology. 2010; No. 2: 46–52.
- Orlova S. P., Lyubarsky E. A., Termalayan N. V., Khozin A. A. Report on the use of the drug Teraflex in the treatment of vertebrogenic pain. 1st neurological department of the Municipal Medical Institution “City Hospital No. 4”, Rostov-on-Don.
- Fairbank J., Mbaot JC, Davies JB, O`Brien JP The Oswestry Low Back Pain Disability Questionnaire // Physiotherapy. 1980. Vol. 66. No. 8. P. 271–274.
N. V. Chichasova, Doctor of Medical Sciences, Professor
GBOU VPO First Moscow State Medical University named after. I. M. Sechenova Ministry of Health of the Russian Federation, Moscow
Contact Information
Special instructions for the use of the drug Teraflex Advance
Do not exceed the recommended dose and duration of use. There are no clinical data regarding the effectiveness and safety of the drug in children under 12 years of age, during pregnancy and lactation. In patients with concomitant liver or kidney diseases, monitoring of liver and kidney functions and hemograms is recommended at the beginning of treatment. Drinking alcohol is not recommended during treatment with Theraflex Advance. Taking the drug does not affect the ability to drive vehicles.
Teraflex: description of the drug
The medicine contains 500 mg of glucosamine hydrochloride and 400 mg of sodium chondroitin sulfate. Also present are stearic acid, magnesium sulfate and stearate, croscarmellose sodium and gelatin.
Useful properties of the drug:
- Restoration of metabolic processes in the cartilage structure.
- Slowing down the destruction of cartilage.
- Restoration of tissue structure.
The medicine is used as part of complex therapy for diseases of the musculoskeletal and musculoskeletal systems. The medication is especially recommended for dystrophic and degenerative changes in the joints. Doctors prescribe pills for the following conditions:
- Osteochondrosis.
- Primary or secondary osteoarthritis.
- Bone injury.
The drug is prohibited for use when:
- Severe renal failure.
- Feeding a baby with breast milk.
- Up to 15 years of age.
- Pregnancy.
- Allergies to chondroitin sodium sulfate, glucosamine hydrochloride and other components.
With caution and under the supervision of a specialist, this medicine should be taken by those people who have diabetes, bronchial asthma, or a tendency to bleed. Studies have been conducted regarding the use of such a drug during pregnancy. But medical scientists have not been able to establish the safety of using the medication during pregnancy.
For children and adults, Teraflex is prescribed twice a day, one capsule at a time . After three weeks of therapy, the regimen is changed: take a tablet once a day for a month. After a 2-3 month break, the course is resumed. A lasting therapeutic effect is achieved after six months of use.
The medication can cause a number of side effects:
- Nausea.
- Lower abdominal pain.
- Tachycardia.
- Intestinal disorder.
- Severe drowsiness.
- Peripheral edema.
- Flatulence.
These unpleasant symptoms usually appear due to an overdose or an allergy to the components of the drug.
Comparison of the effectiveness of Teraflex Advance and Teraflex
Teraflex Advance is more effective than Teraflex - this means that the ability of the drug substance to provide the maximum possible effect is different.
For example, if the therapeutic effect of Teraflex Advance is more pronounced, then it is impossible to achieve this effect with Teraflex even in large doses.
Also, the speed of therapy is an indicator of the speed of the therapeutic action; Teraflex Advance and Teraflex are also different, as is bioavailability - the amount of the drug reaching the site of its action in the body. The higher the bioavailability, the less it will be lost during absorption and use by the body.