Pharmacological properties of the drug Teraflex Advance
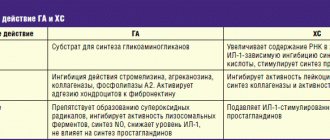
Chondroitin sulfate is a high molecular weight mucopolysaccharide that takes part in the construction of cartilage tissue. Reduces the activity of enzymes that destroy articular cartilage and stimulates the regeneration of the latter. In the early stages of the inflammatory process, chondroitin sulfate reduces its activity and thus slows down the degeneration of cartilage tissue. Eliminates pain, improves joint function, reduces the need for NSAIDs in patients with osteoarthritis of the knee and hip joints. Glucosamine sulfate has chondroprotective properties, reduces the deficiency of glycosamines in the body, and takes part in the biosynthesis of proteoglycans and hyaluronic acid. Having an affinity for cartilage tissue, glucosamine initiates the process of sulfur fixation during the synthesis of chondroitinsulfuric acid. Glucosamine sulfate selectively acts on articular cartilage, is a specific substrate and stimulator of the synthesis of hyaluronic acid and proteoglycans, inhibits the formation of superoxide radicals and enzymes that cause damage to cartilage tissue (collagenase and phospholipase); prevents disruption of glycosaminoglycan biosynthesis induced by NSAIDs and the destructive effect of glucocorticoids on chondrocytes. Ibuprofen has analgesic, anti-inflammatory, and antipyretic effects. The mechanism of action of ibuprofen occurs due to the selective blocking of cyclooxygenase (COX-1 and -2), the main enzyme in the metabolism of arachidonic acid, which leads to a decrease in the synthesis of prostaglandins. Reduces morning stiffness of joints, helps increase range of motion in joints and spine. Pharmacokinetics. Absorption: after a single oral dose of the drug in an average therapeutic dose, the maximum concentration of chondroitin sulfate in the blood plasma is achieved after 3-4 hours, in the synovial fluid - after 4-5 hours. Bioavailability of the drug is 13%. Excretion is carried out mainly by the kidneys within 24 hours. 90% of glucosamine taken enterally is absorbed in the intestine. Over 25% of the dose taken penetrates from the blood plasma into the cartilage tissue and synovial membranes of the joints. In the liver, part of the drug is metabolized to form urea, carbon dioxide and water. The bioavailability of glucosamine is 25% due to primary passage through the liver. The maximum concentration of glucosamine is determined in articular cartilage, liver and kidneys. About 30% of the dose taken persists for a long time in bone and muscle tissue. It is excreted primarily in urine unchanged, and partially in feces. The half-life is 68 hours. Ibuprofen, when administered orally, is almost completely absorbed from the gastrointestinal tract. Eating food at the same time slows down the rate of absorption. Metabolized in the liver (up to 90%). The half-life is 2–3 hours; 80% of the dose taken is excreted in the urine, mainly in the form of metabolites.
Teraflex® Advance
L.RU.MKT.CC.11.2018.2457
* According to the IQUVIA Solutions database “Retail audit of FPPs and dietary supplements in the Russian Federation”, the trade name Teraflex is the leader in sales volume at wholesale and retail prices in group 02G2C “Systemic preparations for joints, in capsules/tablets” (OTC classification) for period July 2017-July 2021.
** 1 capsule 3 times a day (3 weeks), then 1 capsule 2 times a day.
1. It has no analogues in composition according to the GRLS database dated July 27, 2018.
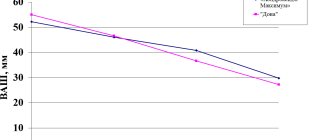
2. Teraflex Advance capsules additionally contain ibuprofen, which provides reliable relief of pain and stiffness after 2 weeks of treatment, compared to conventional Teraflex capsules (2 months). Povoroznyuk V.V. The effectiveness of the drug Teraflex Advance in the treatment of pain in osteoarthritis of the knee joints. Health of Ukraine. 2007; 1–3.
3. Non-steroidal anti-inflammatory drugs without a chondroprotective component are meant.
4. Recommended daily doses when taking Theraflex Advance capsules exceed those of some glucosamine and chondroitin preparations, according to the GRLS database as of July 26, 2018.
5. Teraflex Advance promotes a significant reduction in pain in patients suffering from OA already on the 10th day of therapy. Povoroznyuk V.V., Grigorieva N.V., Gritsenko G.N., Orlik T.V., Dzerovich N.I. and others. The effectiveness of the drug Teraflex Advance: results of a new multicenter study. Newspaper “News of Medicine and Pharmacy” 20 (346) 2010.
6. Tallarida RJ, Cowan A, Raffa RB. Antinociceptive synergy, additivity, and subadditivity with combinations of oral glucosamine plus nonopioid analgesics in mice. J Pharmacol Exp Ther. 2003 Nov;307(2):699-704.
7. Castellsague J. et al. Safety of Non-Steroidal Anti-Inflammatory Drugs (SOS) Project. Individual NSAIDs and upper gastrointestinal complications: a systematic review and meta-analysis of observational studies (the SOS project). Drug Saf. 2012 Dec 1;35(12):1127-46.
8. For combination drugs. Cole G. Evaluating Development and Production Costs: Tablets Versus Capsules. Pharmaceutical Technology Europe; Vol. 5, Pgs. 17 – 26 (1998).
9. Zonova E.V. Osteoarthritis. Choosing a safe treatment strategy for a patient with comorbidity // Effective pharmacotherapy. Rheumatology, traumatology and orthopedics. - 2014. - No. 3. - p.18-22.
10. Federal clinical guidelines for the diagnosis and treatment of osteoarthritis, “Association of Rheumatologists of Russia”, 2021, 19 pages.
Special instructions for the use of the drug Teraflex Advance
Do not exceed the recommended dose and duration of use. There are no clinical data regarding the effectiveness and safety of the drug in children under 12 years of age, during pregnancy and lactation. In patients with concomitant liver or kidney diseases, monitoring of liver and kidney functions and hemograms is recommended at the beginning of treatment. Drinking alcohol is not recommended during treatment with Theraflex Advance. Taking the drug does not affect the ability to drive vehicles.
Teraflex Advance caps No. 60
Dosage
250 mg+100 mg+200 mg
Active substance
Glucosamine + Ibuprofen + Chondroitin sulfate
Manufacturer
Contract Pharmacal Corporation (USA)
Shelf life
2 years
Storage conditions
At a temperature not exceeding 25 °C
Registration certificate number
LS-002678 dated 08/21/2019
Compound
| Capsules | 1 caps. |
| active substances: | |
| glucosamine sulfate (as D-glucosamine sulfate potassium chloride) | 250 mg |
| chondroitin sulfate sodium | 200 mg |
| (in the form of 90% substance, taking into account 10% loss in weight during drying and excess - 241 mg) | |
| ibuprofen | 100 mg |
| (in the form of directly compressed granules 66% (ibuprofen - 66%, pregelatinized starch - 8%, croscarmellose sodium - 2%, MCC - 14%, colloidal silicon dioxide - 1%, stearic acid - 1.5%, corn starch - 6 %, povidone - 1.5%) - 152 mg) | |
| excipients: MCC - 17.4 mg; corn starch (pregelatinized starch) - 4.1 mg; stearic acid - 10.2 mg; sodium carboxymethyl starch - 10 mg; crospovidone - 10 mg; magnesium stearate - 3 mg; silicon dioxide - 2 mg; povidone - 0.3 mg | |
| gelatin capsule: gelatin - 97.07 mg; titanium dioxide - 2.83 mg; brilliant blue dye aluminum varnish - 0.09 mg | |
| ink composition: shellac NF ; ethyl alcohol, dehydrogenated USP ; isopropyl alcohol USP ; butyl alcohol NF ; propylene glycol USP ; ammonia solution NF ; indigo carmine aluminum varnish; titanium dioxide USP |
Characteristic
| Capsules | 1 caps. |
| active substances: | |
| glucosamine sulfate (as D-glucosamine sulfate potassium chloride) | 250 mg |
| chondroitin sulfate sodium | 200 mg |
| (in the form of 90% substance, taking into account 10% loss in weight during drying and excess - 241 mg) | |
| ibuprofen | 100 mg |
| (in the form of directly compressed granules 66% (ibuprofen - 66%, pregelatinized starch - 8%, croscarmellose sodium - 2%, MCC - 14%, colloidal silicon dioxide - 1%, stearic acid - 1.5%, corn starch - 6 %, povidone - 1.5%) - 152 mg) | |
| excipients: MCC - 17.4 mg; corn starch (pregelatinized starch) - 4.1 mg; stearic acid - 10.2 mg; sodium carboxymethyl starch - 10 mg; crospovidone - 10 mg; magnesium stearate - 3 mg; silicon dioxide - 2 mg; povidone - 0.3 mg | |
| gelatin capsule: gelatin - 97.07 mg; titanium dioxide - 2.83 mg; brilliant blue dye aluminum varnish - 0.09 mg | |
| ink composition: shellac NF ; ethyl alcohol, dehydrogenated USP ; isopropyl alcohol USP ; butyl alcohol NF ; propylene glycol USP ; ammonia solution NF ; indigo carmine aluminum varnish; titanium dioxide USP |
Description of the dosage form
Opaque hard gelatin capsules, size No. 0, consisting of two parts - a blue cap and a white body with the blue inscription "THERAFLEX ADVANCE", filled with a white or almost white powder with a slight odor.
Pharmacokinetics
The bioavailability of glucosamine when taken orally is 25% (first pass effect through the liver), the highest concentrations are found in the liver, kidneys and articular cartilage. About 30% of the dose taken persists for a long time in bone and muscle tissue. It is excreted mainly in urine unchanged, and partially in feces. T1/2 - 68 hours.
More than 70% of chondroitin sulfate is absorbed in the digestive tract. Bioavailability is about 13%. With a single oral administration of an average therapeutic dose, Cmax in plasma is observed after 3-4 hours, in synovial fluid - after 4-5 hours. The drug absorbed in the gastrointestinal tract accumulates in the synovial fluid. Excreted by the kidneys.
Ibuprofen is well absorbed from the stomach. Tmax - about 1 hour. Ibuprofen is approximately 99% bound to plasma proteins. It is slowly distributed in and cleared from synovial fluid more slowly than from plasma. Ibuprofen is metabolized in the liver primarily by hydroxylation and carboxylation of the isobutyl group. The CYP2C9 isoenzyme takes part in the metabolism of the drug. After absorption, approximately 60% of the pharmacologically inactive R form of ibuprofen is slowly transformed into the active S form. Ibuprofen has biphasic elimination kinetics. T1/2 from plasma is 2–3 hours. Up to 90% of the dose can be found in the urine in the form of metabolites and their conjugates. Less than 1% is excreted unchanged in urine and, to a lesser extent, in bile. Ibuprofen is completely eliminated within 24 hours.
Pharmacodynamics
Teraflex® Advance is a combination drug containing chondroitin sulfate, glucosamine sulfate and ibuprofen as active ingredients.
Chondroitin sulfate
participates in the construction and restoration of cartilage tissue, protects it from destruction and improves joint mobility.
Glucosamine sulfate
activates the synthesis of proteoglycans, hyaluronic, chondroitinsulfuric acids and other substances that make up the joint membranes, intra-articular fluid and cartilage tissue.
Ibuprofen
is a derivative of propionic acid and has an analgesic, antipyretic and anti-inflammatory effect due to the non-selective blockade of COX-1 and COX-2.
Glucosamine sulfate and chondroitin sulfate contained in the drug potentiate the analgesic effect of ibuprofen.
Contraindications
hypersensitivity to any of the ingredients included in the drug;
history of hypersensitivity to acetylsalicylic acid or other NSAIDs;
erosive and ulcerative diseases of the gastrointestinal tract (including peptic ulcer of the stomach and duodenum in the acute stage, Crohn's disease, ulcerative colitis);
complete or incomplete combination of bronchial asthma, recurrent nasal polyposis and paranasal sinuses;
confirmed hyperkalemia;
hemophilia and other bleeding disorders (including hypocoagulation), hemorrhagic diathesis;
gastrointestinal bleeding and intracranial hemorrhage;
severe renal failure (creatinine Cl less than 30 ml/min), progressive kidney disease;
severe liver failure or active liver disease;
severe heart failure, the period after coronary artery bypass grafting;
pregnancy;
lactation period;
children under 18 years of age.
With caution:
elderly age;
heart failure; arterial hypertension; liver cirrhosis with portal hypertension; liver and/or kidney failure; nephrotic syndrome; hyperbilirubinemia; peptic ulcer of the stomach and duodenum (history); gastritis; enteritis; colitis; blood diseases of unknown etiology (leukopenia and anemia); bronchial asthma; diabetes; concomitant therapy with anticoagulants, antiplatelet agents, corticosteroids, SSRIs; peripheral arterial disease; moderate renal failure (Cl creatinine 30–60 ml/min); smoking; alcoholism; dyslipidemia/hyperlipidemia; cardiac ischemia; cerebrovascular diseases; presence of Helicobacter pylori
; long-term use of NSAIDs; tuberculosis; severe somatic diseases.
If you are intolerant to seafood (shrimp, shellfish), the likelihood of developing allergic reactions to the drug increases.
Use during pregnancy and breastfeeding
Not recommended for use during pregnancy and breastfeeding.
Directions for use and doses
Inside.
Adults take 2 caps. 3 times a day after meals, with a small amount of water. The duration of treatment without consulting a doctor should not exceed 3 weeks. Further use of the drug should be agreed with your doctor.
Side effects
When using the drug Teraflex® Advance, nausea, abdominal pain, flatulence, diarrhea, constipation, allergic reactions are possible; these reactions disappear after discontinuation of the drug.
The possibility of developing adverse reactions associated with ibuprofen present in the drug should be taken into account.
From the gastrointestinal tract:
NSAID gastropathy (abdominal pain, nausea, vomiting, heartburn, loss of appetite, diarrhea, flatulence, constipation; rarely - ulceration of the gastrointestinal mucosa, which in some cases is complicated by perforation and bleeding); irritation or dryness of the oral mucosa, pain in the mouth, ulceration of the gum mucosa, aphthous stomatitis, pancreatitis, exacerbation of colitis and Crohn's disease.
From the hepatobiliary system:
hepatitis.
From the respiratory system:
shortness of breath, bronchospasm.
From the senses:
hearing impairment (hearing loss, ringing or tinnitus), visual impairment (toxic damage to the optic nerve, blurred vision or double vision, scotoma, dryness and irritation of the eyes, swelling of the conjunctiva and eyelids of allergic origin).
From the central nervous system and peripheral nervous system:
headache, dizziness, insomnia, anxiety, nervousness and irritability, psychomotor agitation, drowsiness, depression, confusion, hallucinations; rarely - aseptic meningitis (more often in patients with autoimmune diseases).
From the SSS side:
development or worsening of heart failure, tachycardia, increased blood pressure, increased risk of arterial thrombosis.
From the urinary system:
acute renal failure, allergic nephritis, nephrotic syndrome (edema), polyuria, cystitis.
Allergic reactions:
skin rash (usually erythematous or urticaria), skin itching, angioedema, anaphylactoid reactions, anaphylactic shock, dyspnea, fever, erythema multiforme exudative (including Stevens-Johnson syndrome), toxic epidermal necrolysis (Lyell's syndrome), eosinophilia, allergic rhinitis.
From the hematopoietic organs:
anemia (including hemolytic, aplastic), thrombocytopenia and thrombocytopenic purpura, agranulocytosis, leukopenia.
Other:
increased sweating.
Laboratory indicators:
bleeding time (may increase), serum glucose concentration (may decrease), creatinine Cl (may decrease), hematocrit or Hb (may decrease), serum creatinine concentration (may increase), liver transaminase activity (may increase), urea concentration in blood (may increase), bilirubin concentration in the blood (may increase).
Interaction
Inducers of microsomal oxidation (phenytoin, ethanol, barbiturates, rifampicin, phenylbutazone, tricyclic antidepressants) increase the production of hydroxylated active metabolites of ibuprofen, increasing the risk of severe hepatotoxic reactions.
Microsomal oxidation inhibitors reduce the risk of hepatotoxicity.
Reduces the hypotensive activity of vasodilators (including CCBs and ACE inhibitors), natriuretic and diuretic activity - furosemide and hydrochlorothiazide.
Reduces the effectiveness of uricosuric drugs, enhances the effect of indirect anticoagulants, antiplatelet agents, fibrinolytics (increasing the risk of hemorrhagic complications), ulcerogenic effect with bleeding of corticosteroids, NSAIDs, colchicine, estrogens, ethanol; enhances the effect of oral hypoglycemic drugs and insulin.
Antacids and cholestyramine reduce the absorption of ibuprofen.
Increases the blood concentration of digoxin, lithium and methotrexate.
Caffeine enhances the analgesic effect.
When administered simultaneously, ibuprofen reduces the anti-inflammatory and antiplatelet effect of acetylsalicylic acid (an increase in the incidence of acute coronary insufficiency in patients receiving small doses of acetylsalicylic acid as an antiplatelet agent is possible after starting ibuprofen).
The simultaneous use of drugs containing glucosamine and coumarin anticoagulants (including warfarin) may lead to an increase in INR and the risk of bleeding; it is necessary to monitor blood clotting parameters.
When prescribed simultaneously with anticoagulant and thrombolytic drugs (alteplase, streptokinase, urokinase), the risk of bleeding increases.
Cefamandole, cefoperazone, cefotetan, valproic acid, plicamycin increase the incidence of hypoprothrombinemia.
Myelotoxic drugs increase the manifestations of hematotoxicity.
Cyclosporine and gold preparations enhance the effect of ibuprofen on the synthesis of PG in the kidneys, which is manifested by increased nephrotoxicity.
Ibuprofen increases the plasma concentration of cyclosporine and the likelihood of developing its hepatotoxic effects.
Drugs that block tubular secretion reduce excretion and increase plasma concentrations of ibuprofen.
Due to the glucosamine content in the drug, the effectiveness of hypoglycemic drugs, doxorubicin, teniposide, and etoposide may be reduced.
Glucosamine increases the absorption of tetracyclines and reduces the effect of semisynthetic penicillins and chloramphenicol.
Co-administration with potassium-sparing diuretics increases the risk of hyperkalemia.
NSAIDs may decrease the effect of mifepristone.
Concomitant use of NSAIDs and tacrolimus may increase the risk of nephrotoxicity.
Co-administration of zidovudine increases the risk of hematological toxicity of NSAIDs.
Concomitant use of quinolones and NSAIDs increases the risk of seizures.
Overdose
Symptoms (associated with ibuprofen):
abdominal pain, nausea, vomiting, lethargy, drowsiness, depression, headache, tinnitus, metabolic acidosis, coma, acute renal failure, decreased blood pressure, renal tubular acidosis, hypokalemia, hyperkalemia, bradycardia, tachycardia, atrial fibrillation, respiratory arrest.
Treatment:
gastric lavage (effective only within 1 hour after administration), administration of activated charcoal, alkaline drinking, forced diuresis, symptomatic therapy (correction of acid-base status, blood pressure).
special instructions
During long-term treatment, monitoring of the peripheral blood picture and the functional state of the liver and kidneys is necessary.
When symptoms of gastropathy appear, careful monitoring is indicated, including esophagogastroduodenoscopy, a blood test to determine Hb, hematocrit, and a stool test for occult blood.
If it is necessary to simultaneously take additional NSAIDs and analgesic drugs, the doctor should take into account the presence of ibuprofen in the drug. If long-term use of additional NSAIDs is necessary, Teraflex®, which does not contain ibuprofen, should be used.
If it is necessary to determine 17-ketosteroids, the drug should be discontinued 48 hours before the study.
During the treatment period, alcohol consumption is not recommended.
Impact on the ability to operate machinery and other vehicles.
During the treatment period, you should refrain from driving vehicles and performing actions that require concentration or psychomotor reactions.
Conditions for dispensing from pharmacies
Over the counter.
Pharmaceutical actions
stimulating the regeneration of cartilage tissue, anti-inflammatory, analgesic
Teraflex® Advance (Theraflex advance)
Inducers of microsomal oxidation (phenytoin, ethanol, barbiturates, rifampicin, phenylbutazone, tricyclic antidepressants) increase the production of hydroxylated active metabolites, increasing the risk of severe hepatotoxic reactions.
Microsomal oxidation inhibitors reduce the risk of hepatotoxicity.
The drug reduces the hypotensive effect of vasodilators (including slow calcium channel blockers and ACE inhibitors).
The drug reduces the natriuretic and diuretic activity of furosemide and hydrochlorothiazide.
Reduces the effectiveness of uricosuric drugs.
Strengthens the effect of indirect anticoagulants, antiplatelet agents, fibrinolytics (increasing the risk of hemorrhagic complications).
Strengthens the ulcerogenic effect (with the development of bleeding) of GCS, NSAIDs, colchicine, estrogens, ethanol.
Enhances the effect of oral hypoglycemic drugs and insulin.
Antacids and cholestyramine reduce the absorption of ibuprofen.
Increases the blood concentration of digoxin, lithium and methotrexate.
Caffeine enhances the analgesic effect of Teraflex Advance.
When administered simultaneously, ibuprofen reduces the anti-inflammatory and antiplatelet effect of acetylsalicylic acid (it is possible to increase the incidence of acute coronary insufficiency in patients receiving small doses of acetylsalicylic acid as an antiplatelet agent after starting ibuprofen).
When prescribed with anticoagulant and thrombolytic drugs (alteplase, streptokinase, urokinase), the risk of bleeding simultaneously increases.
Cefamandole, cefoperazone, cefotetan, valproic acid, plicamycin increase the incidence of hypoprothrombinemia.
Myelotoxic drugs increase the manifestations of hematotoxicity.
Cyclosporine and gold preparations enhance the effect of ibuprofen on the synthesis of prostaglandins in the kidneys, which is manifested by increased nephrotoxicity.
Ibuprofen increases the plasma concentration of cyclosporine and the likelihood of developing its hepatotoxic effects.
Drugs that block tubular secretion reduce excretion and increase plasma concentrations of ibuprofen.
Due to the glucosamine content in the drug, the effectiveness of hypoglycemic drugs, doxorubicin, teniposide, and etoposide may be reduced.
Glucosamine increases the absorption of tetracyclines and reduces the effect of semisynthetic penicillins and chloramphenicol.