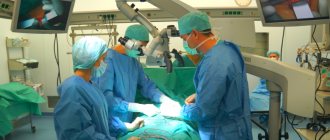
The famous company DePuy Synthes is part of the huge Johnson & Johnson holding. The main specialization is the production of innovative and effective medical equipment in the field of orthopedics, traumatology and surgery.
Delta Motion
DePuy pioneered the production of prosthetic devices in the United States. It was founded at the end of the nineteenth century. At the beginning of their activities, the company’s specialists made a real revolution in the treatment of femoral neck fractures. They stopped using wooden devices that had previously been used to immobilize injured limbs. They were replaced by wire tires, which turned out to be more effective.
Production of the company
DePuy Corporation produces and sells ultra-strong, reliable and modern medical products that are used in the treatment of fractures, sports injuries, endoprosthetics of small and large joints, and so on. The product range consists of the following devices:
- prostheses used for total hip and knee replacement surgeries;
- instruments used in arthroscopic surgeries: fixators and implants;
- medical products that are used during neurosurgical operations: drills, bypass systems, automated monitoring systems and much more;
- systems for fixing limbs in case of injuries and fractures.
Preparing for surgery
In preparation for endoprosthetics, a number of studies and analyzes will be carried out to confirm the need for the operation and eliminate possible contraindications. The patient will also consult with an anesthesiologist.
During your consultation with the surgeon, the most suitable type of endoprosthesis will be selected. The prosthesis and prosthetic technique are selected individually, depending on the degree of wear of the knee joint. If one area of the joint is affected, then partial (unipolar) prosthetics can be performed.
How is the operation performed?
Surgery can be performed under either general anesthesia or spinal anesthesia. It usually takes from one to one and a half hours. The knee joint is accessed through a short skin incision on the inside of the joint. The worn-out articular surfaces are processed according to a special scheme so that an artificial knee joint of the appropriate size can be well fitted to the bone.
What risks are associated with this type of surgery?
Fortunately, complications after such an operation are very rare. Preparation for the operation is thorough, without haste. Thus, the risk and danger of complications are minimized. Despite all the preventive measures during knee replacement surgery, as with any other operation, there are certain risks that occur in less than 5% of cases. The use of good surgical technique and proper handling of the patient with the “new knee” after such an operation gives very good results. With knee replacement, you can again enjoy life without pain and move more. However, be sure to see your doctor for regular checkups in the years to come!
Rules of conduct that contribute to the success of the operation
Below are some important rules of conduct that contribute to the success of the operation. So, it follows:
- After discharge from the hospital, continue performing the learned physiotherapeutic exercises
- step on your foot correctly
- use crutches when walking until stable walking and normal gait are achieved
- maintain a straight gait with steps of equal length
- use lace-up shoes with soft, flexible soles
Delta Motion custom endoprosthesis
DePuy prosthetic devices fully correspond to the anatomical structure of the damaged joint, which allows you to fully restore motor functions after surgery. Medical technology and scientific research do not stand still. The Delta motion endoprosthesis allows you to replace the affected areas of the joint while preserving a significant amount of bone mass.
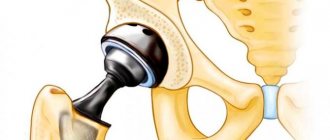
The point of surgery is to install a special medical implant, which consists of three components:
- heads;
- acetabular cup with liner;
- bushings.
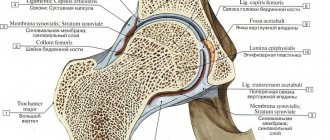
The prosthesis shaft is cemented or pressed into the glenoid cavity. Sometimes screws and special fasteners are used to secure the product. In some other cases, when implanted into the body, the endoprosthesis is supported by ligaments and muscles.
The average lifespan of a modern endoprosthesis is from fifteen to twenty years. After this period, the old prosthesis is replaced with a new one during a repeat operation. As a rule, the date for the next check of the implant’s condition is set directly by the attending physician.
Knee joint prostheses for total arthroplasty
Zimmer - The NexGen® LPS-Flex Mobile Zimmer - LPS-Mobile Bearing Knees
- With a movable platform.
- Both systems allow the artificial knee joint to flex and rotate freely.
- Metal components are used: attached to the end of the femur, replacing the upper part of the tibia.
- Endoprostheses include a plastic articular surface loosely attached to a base plate that acts as artificial cartilage.
- Provide 155⁰ active flexion and 25 degrees of unimpeded internal and external rotation.
- Bone cement is used for fixation.
The Zimmer Gender Solutions® Patello-Femoral Joint
- Knee joint prostheses with a movable platform.
- Designed for active patients, positioned as an early intervention option.
- Allows you to preserve large areas of bone, requiring a smaller incision.
- The use of this implant requires the surgeon to be able to handle special instruments and have knowledge of minimally invasive techniques.
- The endoprosthesis takes into account gender differences; the special shape of its components more accurately reflects the characteristics of female and male anatomy.
- Includes 35 different sizing options.
DePuy Sigma® Rotating Platform Knees
- With a movable platform.
- It involves installing a movable bearing supported on a rotating platform, which allows you to simulate the natural movements of the knee.
- The design ensures a reduction in internal stress and wear, so it lasts longer than its analogues.
- According to statistics, about 1 million such endoprostheses have been implanted in the world, 97% of which have been used for more than 20 years.
DePuy Sigma® Fixed-Bearing Knees
- Fixed support.
- Wear-resistant polyethylene and advanced metal components are used, which do not cause rejection in 99.6% of cases after 5 years of operation.
Smith & Nephew OXINIUM®
- Fixed support.
- Prosthetic knee joint with a high level of wear resistance.
- Made from zirconium metal, which makes it more resistant to abrasion and scratching.
- During testing, an 85% reduction in wear of polyethylene was noted compared to cobalt-chromium materials used in other implants
Stryker Triathlon® Total Knee Replacement System
- Fixed support.
- This type of artificial joint is designed to work with the body.
- Gives good results in returning motor activity after surgery.
- The design of the device allows the implant to bend, expand, and rotate similar to a natural knee
Biomet Vanguard® Complete Knee System
- Fixed support.
- A device that adapts to the specific features of the patient's anatomy.
- Assumes a high degree of flexion (about 145⁰).
- Available in 90 sizes, 10 hip size options.
Service life and materials of manufacture
The overall service life of the Delta Motion endoprosthesis can be influenced by various factors, both individually and in combination. A decrease or increase in service life can be influenced by the patient’s physical activity, his age and weight, bone structure, the presence of chronic diseases, and, of course, the material from which the implant is made.
In order for the device to last as long as possible, the highest demands are placed on consumables for its production. The parts of the prosthesis are manufactured in such a way as to reduce the effect of friction, which causes components to wear out quickly. Resistance to corrosion processes and other negative effects also plays an important role.
The most popular consumables in the production of prostheses are titanium or chrome alloys using basalt. The required strength and reliability are achieved through the hot forging process. The bushings and heads of the prosthesis are also made of ceramics, and the liners contain the strongest polyethylene.
Knee prostheses for partial arthroplasty
BioMet Oxford® Knee
- A device intended for patients with limited (medial) arthritis.
- The prosthesis uses movable plastic bearings and is implanted on only one side.
- Allows you to remove 75% less bone and cartilage, so its installation procedure is less painful and the recovery rate is higher.
- Clinical trials show 98% success after 10 years of wear and 95% after 15 years
DuPuy Sigma® High Performance Partial Knee
- Intended for active patients who require a high degree of flexion but are not yet ready for total arthroplasty.
- Allows you to kneel, squat, and sit cross-legged.
- The surgeon uses any of its components individually to replace the desired knee compartment.
- Preserves the maximum intact area of the native knee.
GET A DOCTOR'S CONSULTATION
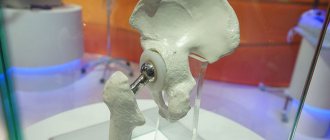
Delta Motion endoprosthesis: description
This system is an uncemented cup with a high-strength ceramic liner. This is a truly innovative solution, since it allows the installation of a prosthesis for young patients whose body does not “get along” well with foreign objects.
The Delta motion design optimizes the head-socket system, allowing larger diameter heads to be used in smaller acetabulums. As a result, the fixation of the implant with the joint becomes better, the patient can lead an active life, resorting to serious physical activity.
The use of the unique Delta motion prosthesis allows you to quickly rehabilitate after surgery and return to a full life. The characteristics of the material ensure the preservation of the natural anatomy of the joint mass; using the product will be completely natural.
The technology of this system makes it possible to produce prostheses specifically designed to suit the characteristics of women's knees. They have a thinner and less voluminous shape, which reduces the load and ensures natural movements.
Main types of knee replacement surgery
Generally speaking, there are two main options for knee replacement surgery:
- Unicondylar endoprosthetics
- Total (complete) endoprosthetics
A unicondylar endoprosthesis replaces only part of the worn-out joint, so this operation is indicated for patients with damage to one condyle of the knee joint.
Total knee arthroplasty can be classified based on the degree of mechanical stability of the endoprosthesis into:
Unrelated
Half-tied
Anatomy of a prosthesis
According to the analytical company GlobalData, by 2021 the global market for total hip replacement will reach $7.1 billion. At the same time, in 2014, the amount of legal claims against the three leading American manufacturers of implants for this operation reached a comparable value of $6 billion. The reason is that that the American regulator FDA for decades allowed the supply of endoprostheses without clinical studies and, even when problems arose, was in no hurry to recall potentially dangerous products from the market.
GlobalData predicts that by 2021, the US will maintain its leadership in the hip replacement market with a 57% share. It is not surprising that most of the litigation with endoprosthesis manufacturers also occurs in America.
The friction pair of an endoprosthesis can be made of different materials: “ceramics-ceramics”, “metal-polyethylene”, “ceramics-polyethylene”, but most complaints and complaints from patients are associated with wearing femoral endoprostheses with a friction pair “metal-metal”.
If mistakes were made at the stages of development and production of such an endoprosthesis, then when the ball rubs against the cup of the artificial joint, too many metal microparticles are formed, which, getting into the tissue surrounding the joint, cause inflammation. In addition, as the joint wears out, it becomes unstable, and the person begins to experience severe pain and discomfort when walking.
In 2012, a study by scientists from the University of Bristol was published in one of the most authoritative medical journals, The Lancet. They analyzed data from the National Joint Registry of England, Wales and Northern Ireland (NJR) and found that metal-to-metal hip replacements deteriorated significantly faster than ceramic ones, for example. Five years after surgery, 6.2% of the first endoprostheses and only 3.2% of the second were subject to replacement.
In the USA, a low-quality prosthesis can be withdrawn from sale by the manufacturer or the FDA, but over the past 20 years the regulator has only
exercised his right to prohibit the supply of low-quality goods. In the vast majority of cases, the sale of endoprostheses was suspended by the manufacturer, but this usually happened when the number of complaints and lawsuits approached a hundred or a thousand. The law requires companies to inform the FDA about recalling products if they may cause harm to health. According to these data, from 2002 to 2013, six leading manufacturers recalled 578 types of femoral endoprostheses and their components from sale.
For example, it took DePuy, the orthopedic division of pharmaceutical giant Johnson & Johnson, three years to remove the ASR system endoprosthesis from sale. Moreover, back in 2007, DePuy engineers tested the internal wear resistance of the system and found that, compared to other endoprostheses, when using ASR, a lot of metal particles get into the thigh tissue. Despite the test results, DePuy continued to sell these hip implants. In 2009, after receiving nearly 400 patient complaints, the FDA asked DePuy to provide additional data on the ASR endoprosthesis, including design clarification and internal testing results. In addition, the FDA informed Johnson & Johnson that high concentrations of metal ions were found in the blood of patients who received DePuy metal-to-metal prostheses. According to the British and Australian national independent registries, ASR endoprostheses showed much lower reliability than stated by the manufacturer. According to NJR, over a five-year period, 13% of people who received the ASR XL system for total hip replacement and 12% of people who received the ASR system for superficial hip replacement (femoral head-sparing) required revision surgery over a five-year period.
Nevertheless, DePuy made the decision to finally withdraw ASR from the market only in August 2010, three years after receiving the first data about the shortcomings of the medical product. By the time sales were discontinued, ASR endoprostheses had been installed in 93 thousand patients worldwide, about a third of whom lived in the United States. In November 2013, Johnson & Johnson announced that it would allocate $4 billion to resolve claims related to the company's defective joints. By June 2014, 12 thousand people filed lawsuits.
More than 300 thousand people around the world (200 thousand of them in the USA) received defective joint endoprostheses from Stryker - Rejuvenate and ABG II. Patients were promised that the devices would last for decades, but for some, problems began after just two years. In June 2012, after receiving an official warning from the FDA, Stryker voluntarily withdrew Rejuvenate and ABG II from sale, advising surgeons that the products could harm hip tissue and lead to more severe complications. In November 2014, the company agreed to pay $1.43 billion to settle claims from patients who received faulty hip replacements. The Stryker agreement included 3,000 lawsuits in Minnesota and New Jersey. Each plaintiff who underwent revision surgery was entitled to a payment of $300,000 or more.
A similar story happened with the Zimmer endoprosthesis. In 2006, the FDA approved their product, the Durom hip component, better known as the Durom Cup, and within a year the first patient complaints appeared. In April 2008, renowned American orthopedic surgeon and Zimmer consultant Lawrence Doerr told the company that the Durom Cup caused excruciating pain in his patients. In response, Zimmer management stated that there was nothing wrong with the endoprosthesis, and Dorr himself was to blame for the patients’ problems because he installed it incorrectly. Under pressure from the FDA, the company suspended sales of the device for several months, citing shortcomings in the instructions for use and installation of endoprostheses. By issuing new instructions for surgeons, Zimmer brought the Durom Cup back to market, where the device remained until the end of 2010. As a result, by 2014, Zimmer paid about $600 million in compensation to victims.
The reason for the problems is that the FDA only began regulating the sale of medical products in 1976. The law established different testing requirements for various medical devices, depending on the potential risk or their role in the life and health of the patient. When the law went into effect, metal-to-metal implants were already successfully sold on the American market, and the FDA decided to consider them “moderate risk” devices. Thanks to this, manufacturers of artificial joints could obtain regulatory approval under a simplified procedure: the company only had to demonstrate that the new endoprosthesis was a version of already sold implants. It was not necessary to conduct expensive clinical trials to prove the effectiveness and safety of the devices.
For 37 years, the FDA turned a blind eye to the endoprosthesis market, and only after a series of scandals broke out in the industry in January 2013, the regulator decided to tighten the requirements and transfer metal-to-metal endoprostheses to the category of “high-risk” products. Today, manufacturers can only obtain FDA approval to sell metal hip replacements after successful clinical trials.
orthopedic market, endoprostheses, endoprosthetics, orthopedics, orthopedic equipment, orthopedic clinic
Subscribe to our channel in Telegram Subscribe to our channel in Yandex Zen
Share on social networks
+1 +1 +1 +1