Spondylitis
is a disease of the spine of inflammatory etiology.
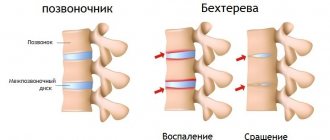
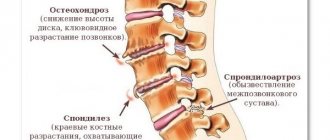
First of all, it affects the vertebral body, destroying it and reducing its height. In severe cases, a hump may form, ossification of the rib joints and displacement of internal organs. But this is not the only danger of spondylitis
.
In the long term, it leads to complications in the heart, liver, kidneys and nervous system. Both old and young are susceptible to the disease. The pathology is often observed in adolescents over the age of 15 years. Fortunately, spondylitis
is not very widespread - no more than 0.6% of people suffer from it.
But if the disease already exists, is it possible to avoid spinal deformation and worsening the condition? Let's understand the nature of spondylitis
.
Most importantly, spondyloarthritis is NOT EQUAL to ankylosing spondylitis!
Spondyloarthritis is a group of inflammatory diseases
characterized by damage to the spine and/or joints. They are grouped according to the mechanisms of their development and similar clinical manifestations. This group is divided into two subgroups - axial and peripheral spondyloarthritis. The difference is that the first affects only the sacroiliac joints and/or spine, while the second can also affect the joints of the upper and lower extremities.
In addition, a subgroup of axial spondylitis is divided into ankylosing spondylitis (this is ankylosing spondylitis) and non-radiographic axial spondylitis. The difference between them is the presence/absence of damage to the sacroiliac joints on radiography. X-ray changes occur very slowly, sometimes it takes 7-8 years for signs of “fusion” to appear, so there is still heated debate about whether these two forms are stages of the same process, or different diseases from the same group.
For a better understanding, below we will talk about the group of axial spondyloarthritis (AS).
Causes of the disease
The exact cause of the disease is unknown.
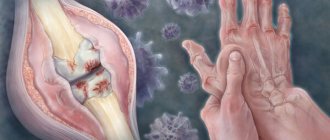
Spondyloarthritis, unlike rheumatoid arthritis, is less of an autoimmune disease and more of a hyperinflammatory disease. They rarely cause systemic inflammatory reactions, but affect certain organs and tissues - joints, skin, intestines, eyes. One of the key factors in the development of AS is the presence of the HLA-B27 antigen. Despite the 40-year history of its study, the role of the antigen remains not entirely clear. According to one theory, the process that triggers inflammation may be microbes in which some of the genes are so similar to the genes of the patient - a carrier of HLA B-27 that the human body “confuses” them and begins to work against its own.
However, when the disease has already manifested itself, do not look for these mysterious microbes - most likely the body has long ago gotten rid of them, and the inflammation will continue.
Another theory is based on the possibility of an abnormal structure of HLA-B27, which itself activates a full-blown immune response. Any factor (trauma, infection) can be a factor—a “trigger”—to activate the “improper functioning” of this antigen. Be that as it may, this notorious HLA B-27 can be detected in 95% of patients with AS.
And then a cascade of reactions unfolds: T-lymphocytes, the cells of the immune system, are sequentially activated and the formation of protein molecules, “inflammatory mediators” (cytokines), increases. They lead to changes characteristic of the entire group of spondyloarthritis and AS in particular. Unlike rheumatoid arthritis, where the main mechanism of joint destruction is the formation of erosions—“eaten away”—of cartilage and bone destruction, in AS the inflammation process ends with bone proliferation—the formation of new “excess” bone tissue. The result is ankylosis (fusion) of the joints and ossification (ossification) of the spinal ligaments, resulting in immobility.
Men are affected more often, with onset occurring before the age of 45 years. It is possible to develop AS in childhood with a transition to adulthood, which is usually quite severe.
Symptoms
The most typical symptom of the disease is pain in the lower back (in the area of the sacrum and on the sides of it) of an inflammatory nature, i.e.
occurring early in the morning or after standing still. The pain can be subtle, like discomfort or a feeling of heaviness, often accompanied by morning stiffness (stiffness), and can radiate to the buttocks, but very often the pain forces the patient to wake up at 4-5 o’clock in the morning.
Symptoms usually progress very slowly (years or even decades), and patients are often observed with a diagnosis of osteochondrosis. The doctor or patient should be alert to the persistent nature of the pain, young age (up to 45 years) and lack of improvement after rest.
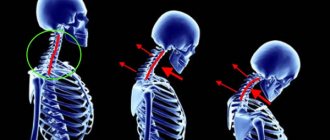
After affecting the sacroiliac joints, AS next affects the vertebrae. Usually the process goes “bottom up”, but less often there may be a different order. In this case, inflammatory changes in the vertebral bodies are first observed, and then intervertebral “bone bridges”—syndesmophytes—develop. Together with calcification of the spinal ligaments, this creates a characteristic “bamboo stick” picture on the x-ray, and during an external examination, the doctor observes the “petitioner’s pose”: the vertebral curves are smoothed, the patient’s head is tilted forward, lateral movements in the spine are possible only by turning the entire torso. The rheumatologist can see such a patient “from afar”, but, unfortunately, at this stage the treatment options are extremely limited. Therefore, modern diagnostic criteria are aimed at the earliest possible diagnosis of AS.
The “classical” instrumental study is an X-ray of the pelvis, which reveals bilateral sacroiliitis - inflammation of the sacroiliac joints. But symptoms on X-rays appear no earlier than after 4-5 years, so for early diagnosis, as the key to effective treatment, the MRI method is used. If sacroiliitis is detected on MRI, but not on radiography, it is called non-radiological. The main symptom of sacroiliitis is swelling of the bone marrow in the area adjacent to the sacroiliac joints.
The laboratory criterion for AS is the HLA-B27 antigen. Remember that, unlike MRI, this is not a sign of a disease, but a marker of PREDISPOSITION to it. Having HLA B-27 is not a death sentence! In general, in the population, HLA B-27 can be found in 8-15% of the population, and only every twentieth of its carriers will have any manifestations associated with this gene.
Other traditional inflammatory changes - an increase in ESR and C-reactive protein - can be observed in only 30% of patients with AS, so normal readings of these tests do not exclude or confirm the presence of the disease.
Unfortunately, the presence of HLA B-27 can be associated not only with AS, but also with damage to other organs. The classic reminder for a rheumatologist is “musculoskeletal system – skin – eyes – intestines.” And how this mosaic will develop in which of the patients is unknown. And since this gene is inherited, one family member may have skin manifestations, while another, for example, may have a combination of AS and intestinal lesions.
In addition to damage to the spine, pathology of the musculoskeletal system associated with HLA B-27 can manifest itself as so-called “enthesitis” - inflammation in the area where the tendon attaches to the bone. The favorite localization in this case is pain in the heel area (on the side or side of the foot) at the insertion of the heel ligament or Achilles tendon.
Another manifestation of the musculoskeletal system may be dactylitis (translated as “inflammation of the finger”), although only the tendons become inflamed, not the joints. In this case, the finger takes on the appearance of a sausage. Inflammation of the joints itself - arthritis: often asymmetrical with greater involvement of the lower extremities, is also a symptom that will direct the doctor to search for HLA B-27. By the way, if the “mosaic” in a particular patient develops in such a way that only the peripheral joints are inflamed without involving the spine, then the disease will acquire the name “peripheral spondyloarthritis.”
Sometimes the manifestation of the disease is anterior uveitis - inflammation of the anterior chamber of the eye. Symptoms: severe pain and redness of the eye, lacrimation, blurred vision (blurred vision), photophobia. A combination with AS may be intestinal damage, accompanied by abdominal pain, weak or loose stools, which may contain mucus or blood. A colonoscopy may diagnose Crohn's disease or ulcerative colitis. Another target organ for HLA B-27 may be the skin, so the doctor will certainly ask about the presence of psoriasis.
Thus, a combination of the patient’s complaints and medical history, conducting specific tests, and analyzing instrumental and laboratory data allows the doctor to diagnose AS and begin therapy.
Autoimmune disease of the inner ear and axial variant of ankylosing spondylitis
Introduction
Hearing impairment, according to numerous clinical and epidemiological studies, occurs in 6–8% of the world's population. At the same time, 68–80% of hearing impairments are sensorineural hearing loss (SNHL) [1]. In 1979, B. McCabe, based on a clinical diagnostic examination and attempts to treat 18 patients, outlined a new clinical symptom complex, which he called autoimmune sensorineural hearing loss. One of the key features of the disease was the rapid positive effect of immunosuppressive therapy (dexamethasone, cyclophosphamide) [2]. Unfortunately, the concept of autoimmunity has not been accepted by the scientific community for a long time. Nevertheless, studies of recent decades have shown that, along with bacterial, viral, vascular and other causes of NCT, a large number of cases of the idiopathic form of the disease are detected. This type of pathology, according to many researchers, can be classified as autoimmune inner ear disease (AIID) [3]. Currently, ABVU is attracting increasing attention from practitioners, since this disease is one of the few potentially curable forms of NST. ABVU usually includes part of idiopathic NST, Meniere's disease, damage to the labyrinth due to otosclerosis, Lyme disease, viral hepatitis, syphilis, damage to the contralateral, previously healthy ear after unilateral laterobasal fractures, all kinds of injuries, including surgical ones, destructive labyrinthitis [4– 6]. Currently, ABVU is divided into 2 main types: type 1 – associated with systemic connective tissue disease (SCTD), type 2 – a localized form of the disease, also observed with concomitant labyrinthitis [7, 8]. The combination of ABVU with CTD occurs in at least 30% of cases. Combinations of ABVU with rheumatoid arthritis, systemic lupus erythematosus (SLE), ankylosing spondylitis, Behçet's disease, ANCA-associated vasculitis (granulomatosis with polyangiitis), relapsing polychondritis, periarteritis nodosa, inflammatory bowel disease (Crohn's disease), etc. have been described [7, 9 -14]. A number of researchers draw attention to the fact that the likelihood of developing ABVD increases with a long course of CTD. For example, with SLE and granulomatosis with polyangiitis, the development of NST at the onset of the disease occurs in 20% of patients, and with a long course, certain manifestations of NST are detected in every second patient [14, 15].
Etiology and pathogenesis
The etiology of ABVU remains unclear, but currently, by analogy with other autoimmune diseases, several theories of their development have been put forward. Accidental damage.
Damage to the inner ear causes the release of cytokines, which delay the development of an additional immune response.
This theory can explain the exacerbation/remission cycle in diseases such as Meniere's disease. The mechanism of molecular mimicry.
Antibodies or migrating T cells cause sudden damage to the inner ear because ear antigens are similar to potentially pathogenic substances, viruses or bacteria.
Currently, this theory is the most popular [16]. Violation of tolerance.
The ear, like the eye, is probably only a partially immunoprivileged locus.
The body may not be aware of all antigens in the inner ear, and when they are released (after surgery or an infection), the immune system reacts to them as if they were a foreign antigen [17]. Genetic factors.
Genetic aspects of the immune response may be associated with increased susceptibility to common hearing diseases such as Meniere's disease. A systematic review of the literature from 1961 to 2011 revealed that about 1/3 of all cases of Meniere's disease are clinically associated with immunological causes and have a positive response to GCS therapy. There are studies that show that patients with Meniere's disease with the major histocompatibility complex (MHC) allelic variant MICA*4 have slower progression of hearing loss than patients with other types of MHC. In confirmed cases of autoimmune sudden hearing loss, cochlin, the most significant protein in the tissues of the inner ear, can induce a T-cell response and is considered an antigen responsible for inflammation and damage to the inner ear [18]. In addition, indirect evidence includes a family history of autoimmune diseases, combination with other autoimmune diseases (SLE, Behçet's disease, granulomatosis with polyangiitis, relapsing polychondritis), frequent detection of certain alleles of the major histocompatibility complex (DR4-, cw7+, cw4+, B35), increased IgG antibody titers and clinical response to immunosuppressive therapy. In patients with ABVU, evidence of the disease such as the presence of a mononuclear infiltrate or deposition of antigen-antibody complexes in the inner ear cannot be identified due to the impossibility of performing a biopsy of the inner ear, although such signs are detected in animal models of the disease.
Epidemiology
The prevalence of ABVU is completely unknown, because there are no specific diagnostic criteria, as well as due to insufficient awareness of doctors. However, ABVD is less common than sudden deafness, with an incidence of 1 in 5,000 to 10,000 people per year. Like other autoimmune diseases, ABVD is observed more often in women, usually manifests itself between the ages of 20 and 50 years, and is rare in childhood [19]. Approximately 50% of patients diagnosed with ABVU have some manifestation of Meniere's syndrome [20].
Clinic
The clinical picture of the disease is nonspecific and can include symptoms of almost all diseases of the inner ear. ABVU most often looks like a rapidly progressive, asymmetrical, often bilateral, fluctuating hearing loss with an atypical (bass) configuration of audiograms, accompanied by noise, a feeling of fullness in the ears, fullness and often dizziness. Less commonly, at the onset of the disease, ABVU may be unilateral. The results of audiological studies (speech audiometry, otoacoustic emissions, evoked and microphone potentials, etc.) may not correspond to the degree of hearing loss according to pure tone threshold audiometry [21]. Speech intelligibility is particularly affected [22, 23]. Dizziness is observed in almost 50% of cases, which causes certain difficulties in differential diagnosis with Meniere’s disease [24]. If the correct diagnosis cannot be established in time and, accordingly, adequate therapy cannot be started, ABVU can spread to both ears and progress to deafness.
Diagnostics
A quick diagnosis and the prescription of adequate therapy determine the auditory prognosis in patients with ABVU. This fact contributes to the search for specific markers of inflammation of the inner ear. Detection of autoantibodies is usually the first step in determining the autoimmune nature of the disease. Unfortunately, specific and sensitive immunological markers of ABVU have not yet been identified [25]. During the study of ABVU, a huge number of diagnostic tests were proposed: antibodies to type 2 collagen, endothelial cells, sulfaglucoronosyl glycolipids, peripheral myelin basic protein 0, etc. The significance of immune complexes and changes in lymphocyte populations in peripheral blood was also studied [26] . The best-studied autoantibody that binds a 68-kDa antigen isolated from bovine temporal bone extract is the inducible form of heat shock protein 70 (HSP-70) [27]. HSP-70 is expressed in various pathologies of the inner ear and is an early marker of cell damage, but it is not specific. In a study by Yeom R. et al. (2003) the frequency of detection of antibodies to HSP-70 did not differ between patients with ABVU and the control group, therefore, HSP-70 cannot be used to diagnose ABVU [28]. Mice immunized with HSP-70 and producing antibodies to HSP-70 do not experience hearing loss, suggesting that these antibodies are not directly involved in the pathogenesis of ABVD. However, these antibodies can be used as markers of disease activity and markers of response to therapy. Autoantibodies to specific cochlear antigens have low specificity for rapidly progressive sensorineural hearing loss. The diagnosis of ABVU is complicated by the impossibility of performing a tissue biopsy of the inner ear, since this manipulation leads to the destruction of the organ and irreversible loss of its function. Thus, at present, the diagnosis of ABVU is based primarily on the characteristics of the clinical picture, signs of progressive sensorineural hearing loss on repeated audiograms, and the response to immunomodulatory drugs, such as corticosteroids. For the diagnosis of ABVU, diagnostic criteria are proposed, presented in Table 1. The presence of ABVU is likely in the presence of 3 major or 2 major and 2 minor diagnostic criteria [25]. When identifying signs of ABVD, it is extremely important, in addition to excluding infectious and vascular causes of the disease, to actively search for systemic autoimmune diseases.
Treatment
Effective treatment of NST of viral or vascular etiology is possible only in the acute period - up to 2 weeks. from the onset of the disease. After this period, regardless of the cause and treatment, hearing, as a rule, does not improve. With ABVU, the therapeutic window can be extended to ≥3–6 months. In this regard, treatment of ABVU should be carried out regardless of how long ago the disease occurred [7, 29, 30]. Corticosteroids
(GCS).
They are the drugs of choice in the treatment of ABVU. Initiation of treatment: a dosage regimen of 1 mg/kg/day of prednisolone or 6-methylprednisolone is used for 1 month. When using shorter courses and lower dosages, effectiveness decreases and the risk of relapse increases [15]. In rapidly progressive forms, the dosage of 1 mg/kg/day is maintained until a stable audiogram is achieved, with a dose reduction over the next 8 weeks. up to 10–20 mg/day. The reduced dosage achieved is maintained for the next 6 weeks. In case of severe hearing loss (more than 70 dB), pulse therapy is performed: 3 pulses of 500 mg each with a transition to oral administration according to the previously indicated regimen. It should be noted that in some patients, hearing improvement is observed only by the 4th week. from the start of therapy. Some patients do not respond to corticosteroids or require higher dosages to control the disease. In such cases, other immunosuppressants such as methotrexate and cyclophosphamide have been attempted. The empirical basis for their use in certain cases is the fact that they potentiate the effect of GCS, thereby achieving remission of one or more symptoms that was not achieved when using GCS in isolation. Immunosuppressants may also be prescribed for steroid-sparing purposes. The average response rate to GCS therapy is 60%. In most patients, the dose of GCS can be reduced until completely discontinued without relapse of the disease, however, in a number of patients, steroid-dependent hearing loss develops. Steroid resistance may also develop, which requires the use of other immunosuppressants. Methotrexate
.
A meta-analysis did not reveal any advantages when prescribing combination treatment compared with GCS monotherapy [31]. However, for dizziness or unsteadiness when walking, longer treatment may have been required. The most frequently used dose of methotrexate was 7.5 mg/week. After achieving a response, the drug was administered orally at a dose of 15 mg/week. for a period of 12 months. Cyclophosphamide
.
Cyclophosphamide was originally used by B. McCabe in his first study [2] and was considered the drug of choice. However, due to its side effects, the drug is not used often and is mainly used in patients resistant to GCS therapy. Oral cyclophosphamide is prescribed at a dose of 1–2 mg/kg/day for 4–6 weeks. When administered intravenously, the initial dose is 0.75 mg/m2 or, in the case of a GFR reduced by more than 1/3 of the norm, 0.5 mg/m2. Injections are performed every 1–3 months. Careful monitoring of peripheral blood leukocytes and neutrophils is necessary. With the simultaneous administration of GCS and cyclophosphamide, co-trimoxazole can be prescribed for the prevention of Pneumocystis pneumonia. Plasmapheresis
.
In a long-term study of patients with ABVU, 50% of patients showed improvement or stabilization of hearing indicators during plasmapheresis [32]. In the study by T.A. Sichkareva (2010), which included 121 patients with acute or chronic NCT, a positive effect in acute NCT was achieved in 73.3% of cases, in chronic NCT – in 59% [33]. It is necessary to note the advisability of using this type of therapy in combination with GCS to avoid the development of rebound syndrome. Intratympanic therapy.
The use of intratympanic corticosteroids is an attractive method of therapy due to its minimal invasiveness, targeted action of the drug on the affected ear, and reduced systemic side effects of corticosteroids.
However, no general recommendations have been developed regarding the dosage and duration of such treatment. A feature of intratympanic therapy is the inability to control exactly how much of the drug reaches the inner ear (part of the drug is absorbed in the middle ear, part is excreted through the Eustachian tube). Thus, the effectiveness of this therapy cannot be fully determined [34]. Etanercept
.
The results of the first attempts to use etanercept are promising, but not conclusive due to the small number of studies conducted [35]. There is evidence of the successful use of etanercept in combination with methotrexate, which may make it possible to refrain from prescribing corticosteroids [36]. The standard dosage is 25 mg subcutaneously 2 times a week. for an indefinite period of time. Adalimumab is prescribed subcutaneously at a dose of 40 mg every 2 weeks. For undefined period. Dosage may be increased to 40 mg/week if decreased response is observed. The drug was successfully used in 1 patient with autoimmune sensorineural hearing loss and rheumatoid arthritis [37]. Infliximab
.
The standard protocol for the use of the drug is a slow intravenous administration (about 2 hours) at a dose of 3 mg/kg at the beginning, at the 2nd and 6th weeks. treatment with a transition to a maintenance regimen every 8 weeks. Intratympanic administration of infiliximab can be used for steroid-sparing purposes in patients with ABVU [38]. Anti-B cell therapy.
Several studies have demonstrated the successful use of rituximab in patients with ABVU [39, 40].
Additional research is required to form a final opinion. The recommended dosage regimen is 1000 mg intravenously, followed by repeated administration of 1000 mg after 2 weeks. Cochlear implants
are used for ABVU at the final stage of the disease [41]. They are extremely expensive, but nevertheless have no analogues for complete deafness. They can be an important alternative to immunosuppressants due to the absence of side effects such as the development of serious infections and cancer. In most cases, a control audiogram is performed on the 2nd week. from the start of therapy. An improvement in hearing sensitivity after treatment is considered significant when the sound perception thresholds decrease by 10–15 dB at at least 3 frequencies.
Clinical observation
A 33-year-old patient
was admitted to the department as planned, for the first time with complaints of inflammatory pain in the cervical, thoracic spine, shoulder joints, fever up to 37.2 °C without chills, catarrhal symptoms; progressive bilateral hearing loss. For many years I have been experiencing mild/moderate inflammatory pain in the cervical and thoracic spine. A diagnosis of dorsopathy was made. In May 2013 (3 months after the birth of the second child) - an attack of systemic dizziness, severe hearing loss in the left ear, lasting about 1 week. with further gradual partial improvement over the next 3 weeks. In August 2014, there was a repeated attack with hearing loss in the right ear, with further recovery over the course of 1 month. Deterioration in the fall of 2015 – progressive hearing loss in both ears. In January – February 2016, she was examined at the City Clinical Hospital at her place of residence. In the general blood test: leukocytes – 14.2×109/l, erythrocytes – 4.77×1012/l, hemoglobin – 132 g/l, hematocrit – 35.9%, platelets – 337×109/l, ESR – 14 mm/h, total protein – 71 g/l, creatinine – 75 µmol/l, urea – 5.2 mmol/l. In the general urine analysis: no protein was detected, leukocytes - 0-3-5 in the visual field, red blood cells, casts were not detected. MSCT of the temporal bones of the paranasal sinuses, X-ray of the chest organs - without pathological changes. Audiometry: bilateral sensorineural hearing loss, grade 4. right, 2 tbsp. left. During therapy with dexamethasone (doses unknown), there was a positive trend in the form of improvement in general well-being, hearing, and reduction in tinnitus. MRI dated February 17, 2016: signs of conical narrowing and decreased blood flow in the left vertebral artery of segments V3 and V4; signs of decreased blood flow in the left vertebral artery in segments V3 and V4. About 1 month before admission - increased pain in the joints, increased temperature to 37.2 ° C without chills, catarrhal symptoms (I had not measured the temperature previously). In laboratory tests for 1 month. before admission (outpatient): leukocytes – 11.63–7.69×109/l, neutrophils – 7.51–4.16×109/l, hemoglobin – 120–122 g/l, erythrocytes – 4.72–4 .72×1012/l, hematocrit – 37.7–37.9%, platelets – 354–330×109/l, ESR – 17–16 mm/h; CRP – 17.1–20.3 mg/l, IgG – 9.7 g/l, IgA – 1.47 g/l, IgM – 1.6 g/l, RF, ANF, C3, C4, calcium, phosphorus, parathyroid hormone, osteocalcin – within normal values. HSV types 1, 2, 6 (DNA) – not detected. HLA-B27 antigen (ELISA) – detected. Consulted by a rheumatologist. At the time of admission, his condition was relatively satisfactory. Increased nutrition, weight 80 kg, stable. Temperature – up to 37.1 °C. Lymph nodes accessible to palpation are not enlarged. In the absence of additional NSAIDs, there is minimal arthritis of the shoulder joints. The skin over them is not changed. Morning stiffness in the cervical and thoracic spine until lunchtime. Hand strength is not reduced. The symptom of compression of the hands and feet is negative on both sides. Benign joint hypermobility syndrome (5 points according to Beighton criteria). Flattening of the physiological curves of the spine, slight scoliosis of the thoracic region with secondary hypertonicity of the rectus muscles. Tension symptoms are negative. Palpation of paravertebral points is painless. Secondary hypertonicity of the muscles of the collar zone. No myalgia. Slight palpation tenderness of the right iliosacral joint. Chest excursion – 4 cm. Chin-sternum test – 1 cm. Ott’s and Thomayer’s symptoms – normal. The temporal arteries are visually unchanged, pulsation is preserved, palpation is painless. In laboratory tests: clinical blood test: hemoglobin – 125 g/l, erythrocytes – 4.99×1012/l, leukocytes – 13.8×109/l, platelets – 380×109/l, ESR – 17 mm/h ( according to Westergren). Blood immunology: CRP – 9.9 mg/l (0-1 mg/l), ANF – 1:320, homogeneous, RF – 10.3 (≤15), p-ANCA – 1:320, c-ANCA – no, antibodies to ds-DNA – no, IgG – 8.2 g/l, IgA – 1.47 g/l, IgM – 1.56 g/l, cryoglobulins – no. Clinical urine analysis - proteinuria 130-150 mg/l. According to the X-ray examination, signs of spondylitis of the cervical and thoracic spine, enthesitis of the ischial and iliac bones were revealed. No signs of damage to the iliosacral joints or destructive damage to peripheral joints were found (including MRI with IV contrast). Also, during gastro- and colonoscopy with biopsy, no data was obtained on the presence of inflammatory bowel diseases in the patient (UC, Crohn's disease). A weakly positive reaction to amyloid was detected at the level of the lamina propria of the small intestine. The audiogram shows signs of stage 4 sensorineural hearing loss. right, 2 tbsp. left. MSCT of the chest did not reveal focal or infiltrative changes in the lung tissue. Thus, during the examination, the clinical, laboratory and instrumental picture indicates that the patient has reliable (according to ASAS criteria) HLA-B27-positive axial spondyloarthritis associated with autoimmune disease of the inner ear. The course of the disease is complicated by the development of bilateral sensorineural hearing loss. Taking into account the results of a colon biopsy and the presence of slight proteinuria, the presence of secondary amyloidosis cannot be excluded, which requires further careful follow-up. Data on the presence of active infectious, specific, paraneoplastic processes, inflammatory bowel diseases, systemic vasculitis (including ANCA-associated) have not yet been received. Taking into account the rate of progression of sensorineural hearing loss noted while the patient was in the department, in the absence of absolute contraindications, a decision was made to conduct pulse therapy with methylprednisolone (250 mg No. 3) followed by a transition to its oral administration (60 mg prednisolone equivalent). The treatment was tolerated satisfactorily; no significant adverse reactions were noted. During therapy, there was an almost complete disappearance of arthritis, inflammatory pain in the cervical and thoracic spine, the severity of tinnitus and peripheral dizziness decreased, which made it possible to expand the patient’s motor mode. Subjectively, the patient also noted a significant improvement in hearing. The control audiogram shows moderate positive dynamics. In control laboratory tests - normalization of the concentration of C-reactive protein, an increase in red blood counts. After 1 month A dose reduction of corticosteroids to a maintenance dose (7.5 mg prednisolone equivalent) was started. Against this background, over the next 6 months. The patient's condition remains stable.
Conclusion
ABVU is a relatively rare disease, the diagnosis of which is quite complex and requires a targeted search for CTD. However, timely initiation of aggressive immunosuppressive therapy preserves hearing in most patients.
Treatment
The patient needs to obtain as much information as possible from the doctor and strictly follow clinical recommendations.
Despite the fact that the list of groups of drugs that affect the course of the disease is small, it is quite possible to avoid ankylosis of the spine and the development of extra-articular complications from other organs and systems. A very important role is played by daily gymnastics, which is indicated for absolutely everyone, regardless of the activity of the disease and the development of ankylosis. Its goal is to slow down the progression, prevent and treat deformities, and improve overall well-being. The main exercises are stretching the spine and strengthening the paravertebral muscles. If we evaluate the arsenal of drugs proven against AS (Attention! Now we are talking only about the spine), then there are two main classes of drugs.
First of all, these are non-steroidal anti-inflammatory drugs (NSAIDs). They should be prescribed to the patient immediately after diagnosis, regardless of the stage of the disease, should be taken for a long time and without interruptions, and can not only reduce pain, but also slow down the progression of AS.
In other words, the fusion of vertebrae with each other occurs four times slower compared to those patients who used NSAIDs only “on demand”. The selection of NSAIDs is carried out by the attending physician, taking into account many factors, including the patient’s concomitant diseases, the characteristics of the drug’s purpose and its possible side effects.
Genetic engineering therapy
– these are TNF-α blockers (infliximab, golimumab, adalimumab, certolizumab pegol, etanercept) and antibodies to interleukin-17 (secukinumab, netakimab). Effective at any stage of AS development (but at an early stage more than at a late stage) both for reducing activity and for preventing deformities. As a rule, they are prescribed if the effect of NSAIDs is insufficient. Taking into account the availability of several drugs from the group of genetic engineering therapy, the doctor has the opportunity to “switch” the patient to another drug if the first one is ineffective.
In the event that extra-articular lesions of the eyes, intestines or skin occur, the doctor’s arsenal in terms of effective therapy expands even further.
Severe complications arise either when the patient presents late or when he ignores the recommended treatment.
Nowadays, therapy allows us to avoid the rapid and pronounced progression of ankylosing spondylitis and make the classic “supplicant pose” a thing of history.
Drugs for spondylitis
Drug therapy is the main treatment for spondylitis. In addition to antibiotics to eliminate the underlying disease, nonsteroidal anti-inflammatory drugs (NSAIDs) are considered the leading drugs. They allow you to relieve the symptoms of inflammation, remove swelling and completely or partially eliminate pain. NSAIDs cannot be taken constantly - they are prescribed only during exacerbations, since they negatively affect the gastrointestinal mucosa.
For severe inflammation and extensive necrosis of spinal tissue, glucocorticoid steroids are prescribed. This group of drugs is also intended for short-term symptomatic use, since long-term use can only worsen spondylitis.
In case of infectious intoxication of the body, drip intravenous administration of solutions to remove toxins is indicated. To protect healthy tissues and improve metabolic processes, B vitamins, blood microcirculation correctors, and vasoprotectors are used.
Due to the fact that the affected spine cannot perform its supporting function in full, the back muscles take on part of the load. Constant overexertion causes severe spasms; to relieve them, patients are prescribed antispasmodics and muscle relaxants.
The only group of medications that really helps restore damaged tissues are chondroprotectors.
Chondroprotective agents for the treatment of spondylitis help accelerate regeneration processes in the spine, protect healthy cells from the adverse effects of toxins and enzymes, and also significantly slow down the progression of the disease. They are most effective in cases of slow or worsening disease, as well as in the recovery period after treatment of septic spondylitis. For spondylitis of various etiologies, doctors recommend a course of Artracam. It is enough to take this chondroprotector in a sachet for 2-4 months a year to prevent or slow down the transition of spondylitis to new joints. Artracam reduces pain, helps prolong remission, and most importantly, allows you to less frequently resort to taking anti-inflammatory drugs that are unsafe for health. Being a natural product based on glucosamine, Artracam has no contraindications for use at any age. And may spondylitis spare your joints!