Monoclonal antibodies are the latest advances in medicine, which are used in the treatment of serious diseases. Among them are malignant neoplasms, autoimmune, systemic, diseases of the cardiovascular system, some infections and much more. In addition, monoclonal antibodies are widely used in diagnostics, for example, in immunohistochemistry, enzyme-linked immunosorbent assay, flow cytometry, etc. Thus, this technology is used in many branches of modern medicine.
- Methods for producing monoclonal antibodies
- Mechanism of action of monoclonal antibodies
- Drugs with monoclonal antibodies
- Problems with the use of monoclonal antibodies
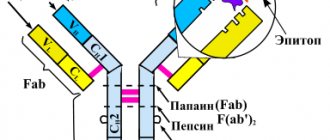
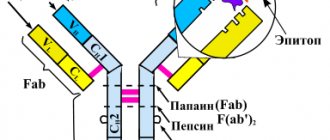
Humanity has long discovered the effect of antibodies - special molecules that are produced by cells of the immune system to recognize foreign agents - antigens and destroy them. Antibodies are specific. This means that they recognize only their own antigen, and not just an antigen, but a separate fragment of it - a determinant group. One antigen may contain several such determinant groups, and different antibodies will be formed to them. Moreover, several types of antibodies can be formed to one determinant at once, which may differ in structure, degree of relatedness and binding strength. Thus, when an antigen is introduced into the body, a large number of different types of antibodies are formed, directed exclusively at one type of antigen. This allows for adequate immune protection.
Antibodies are produced by special antibody-forming cells. Moreover, each type is formed by a separate group of genetically homogeneous cells - clones. The more types of antibodies needed, the more clones are formed. Accordingly, antibodies that are produced by one clone of cells are called monoclonal antibodies.
Previously, to produce antibodies, immunization of animals was used, after which their plasma was selected and used to prepare individual preparations - immune sera to combat various toxins (diphtheria, tetanus), viruses, poisons, etc. But there are situations when a specific antibody is needed, aimed at specific antigen determinant. Here, regular immunization is no longer enough. More targeted technologies are required.
Book a consultation 24 hours a day
+7+7+78
Mechanism of education and work
The final stage of production is growing crops in bioreactors.
The creation of monoclonal antibodies involves the following steps:
- Mice (or other animals that are suitable for this procedure) are immunized by introducing a foreign substance - an antigen.
- After a few weeks, their immune response is checked. This is indicated by the appearance of antibodies to the administered antigen.
- If the result is positive, the mice's spleens are dissected and prepared for cell collection. To do this, the organ is crushed after being washed in distilled water. In a special apparatus, the resulting mass is shaken to separate the cells that will be needed in the end.
- Among the cell homogenizate, T- or B-lymphocytes are found, from which the production of the desired antibodies is expected.
- These cells are mixed with an extract containing cells from the spinal cord affected by a tumor (myeloma). Their ability to mutate activates B lymphocytes.
- Enzymes are added to the resulting suspension and incubated in certain chemical reagents.
- The resulting hybrid cells are grown on nutrient media.
- Using an enzyme immunoassay, the ability of newly created cellular structures to perform their function is tested.
- Selected cell clones are frozen. They are ready for use for medicinal purposes.
Less common methods for preparing monoclonal antibodies are growing them directly in the body of a mouse, inserting a piece of the gene of human immune cells into the genotype of a virus that infects bacteria (bacteriophage), and further genetic engineering manipulations. All MATs have similar mechanisms of operation. They consist of searching for specific antigens that cause the disease and neutralizing them.
Return to contents
What is migraine
Migraine is a chronic neurological disease characterized by prolonged, pulsating headaches of high intensity.
Women suffer from migraines more often than men. As a rule, the first episodes occur in adolescence and young adulthood and continue until the onset of menopause. However, it is possible to develop a first migraine episode at any age. Statistically, in 70% of patients migraine is hereditary. Migraine is characterized by throbbing, pressing, debilitating, usually one-sided headaches. The pain can be localized around the eyes, in the temporal region and forehead. Often, a migraine attack is preceded by a so-called aura - visual, sound, tactile symptoms - lightning, flashes, tingling, numbness.
Indications for use
Prescribed in the treatment of dermatological diseases.
Monoclonal antibodies are used in the following branches of medicine:
- Hematology. For the treatment of diseases of erythrocyte, platelet and leukocyte blood germs.
- Oncology.
- Rheumatology. For the treatment of rheumatoid arthritis and other diseases of an autoimmune nature.
- Neurology. They help treat multiple sclerosis.
- Pulmonology.
- Dermatology. Psoriasis is being treated.
- Transplantology. To inhibit the graft rejection reaction.
The mechanism of action of MAbs in rheumatoid arthritis is based on their ability to reduce the possibility of antigen presentation, inhibit the expression of cytokines - mediators that transmit intercellular commands, and also produce autoantibodies.
Return to contents
Antibody drugs
When diagnosed with psoriasis, monoclonal antibodies are prescribed only by a doctor. In mild forms of the disease, when less than 10% of the epidermis is affected, such medications are not used.
The following drugs are widely used for psoriasis:
- Remicade;
- Stelara;
- Humira.
Each drug has its own characteristics of use, as well as contraindications and side effects. The choice of drug is made by the doctor, depending on the clinical picture of psoriasis in a particular patient.
The specific drug is selected by the doctor, treatment is carried out in a hospital
Remicade
The drug is intended for intravenous drip administration. The main active ingredient is infliximab, a monoclonal hybrid of mouse and human antibodies. 10 ml of the drug is mixed with a physical solution (sodium chloride) and administered intravenously over two hours. The exact dosage and duration of the procedure depend on the characteristics of the patient’s disease symptoms.
Treatment of psoriasis is carried out in several stages. Two weeks after the first dropper, the second dose of the medicine is indicated. The next intravenous administration is carried out after a month and a half. In the future, it is necessary to administer one dose of the medicine every 2-3 months. The procedure is performed only in a hospital.
The drug is indicated for the treatment of psoriasis and psoriatic arthritis. Used for moderate to severe severity of the disease. Additionally, the medicine can be used to treat patients with contraindications to physiotherapy, in particular with intolerance to ultraviolet exposure.
The price of one dose of the drug is about 40 thousand rubles.
Contraindications:
- intolerance to the components of the composition;
- acute heart failure;
- severe infectious diseases;
- childhood;
- hepatitis;
- oncology;
- multiple sclerosis.
The drug is contraindicated in pregnant women, during lactation and in patients under 18 years of age.
The drug is administered through a drip
Stelara
Dermatologists often recommend treating psoriasis and psoriatic arthritis with Stelara. Its peculiarity is that it contains only human antibodies, without any admixtures of animal proteins. Thanks to this composition, the medicine is less likely to cause side effects, unlike its analogues.
The drug is available in a solution for subcutaneous injection. The cost of one dose of medicine is about 160 thousand rubles.
Application regimen: 1 injection every 4 months. Repeated administration of the drug is practiced a month after the first dose, then injections are given every 4 months.
Contraindications are largely similar to the precautions for using Remicade. The medicine is not recommended for the treatment of older patients
In addition, the drug is contraindicated in acute bacterial, infectious or fungal diseases.
The peculiarity of the drug is its rapid therapeutic effect. After the second injection, more than 70% of the epidermis is cleared. Repeated use of the medicine, after 4 months, allows you to stop the development of the disease and increase the duration of remission. Patients note that despite the high cost, treatment with the drug shows really good results and reduces the frequency of exacerbations.
Stelara does not contain components of animal origin, so side effects are less common
Humira
Adalimumab is the active ingredient in Humira. Its action is directed against tumor necrosis factor. The drug is used in the treatment of moderate and severe psoriasis, as well as for joint damage.
The drug is administered subcutaneously every two weeks. It is used as part of complex therapy, or as an independent means to prevent exacerbations. The cost of the medicine is about 35 thousand rubles per dose. There are cheaper domestic analogues, but they are rarely found on sale.
Contraindications are the same as for other drugs in this group.
Like other monoclonal drugs, Humira is used in the treatment of psoriasis and oncology
Method of using monoclonal antibodies for rheumatoid arthritis
The therapeutic course of monoclonal antibodies for rheumatoid arthritis is quite long. The drug is administered intravenously, by drip. The rheumatologist determines the dose of monoclonal antibodies after receiving the results of analyzes of the antigenic composition of the synovial fluid of inflamed joints. They are selected individually for each patient. The introduction of MAT allows you to stop taking non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids (GCC), the use of which has many side effects.
- Experience with the use of rituximab in the treatment of rheumatoid arthritis
Return to contents
Classification of drugs
Before using the drug, a thorough diagnosis and consultation with a doctor is necessary.
Monoclonal antibodies are divided according to the principle of belonging to T- or B-cells of the immune system:
- Followers of T-lymphocytes: Natalizumab, Daclizumab, Alemtuzumab, Ustekinumab.
- B-lymphocyte clones: “Rituximab”, “Ocrelizumab”, “Ofatumumab”, “Accept”.
The most common classification of monoclonal antibodies is based on the methods of their production:
- Mouse. As a result of preparing the drug, 100% of the protein from the cells of these animals is used.
- Chimerical. The protein component obtained from mice makes up 25% of the total concentration.
- Humanized. This figure ranges from 5 to 10%, the remaining proteins belong to humans.
- Human. 100% comes from people.
Mouse, chemical and humanized monoclonal antibodies make up one drug - Infliximab. Among human MAbs, there are several drugs used to treat rheumatoid arthritis - Adalimumab, Golimumab. Monoclonal antibodies are the “gold standard” in the fight against arthritis and other rheumatological and autoimmune diseases.
catad_tema rheumatoid arthritis ArticlesComments
Proceedings of the expert meeting on the registry of patients with rheumatoid arthritis receiving rituximab (MabThera):
Published in the journal: “Scientific and Practical Rheumatology” 2008, supplement to No. 1, pp. 11-14
The pathology of T-cell immune responses has been considered central to the development of RA for several decades. However, in recent years it has been found that B cells can participate in the pathogenesis of RA not only as producers of autoantibodies (in particular, rheumatoid factor - RF), but also as antigen-presenting cells - presenting arthritogenic autoantigen to T cells. As a result, T cells are activated and produce proinflammatory cytokines. The important role of B cells in the development of the rheumatoid process is also confirmed by the therapeutic effect of the anti-B cell drug rituximab in RA.
Rituximab is a chimeric monoclonal antibody against the CD20 molecule, which is found on the surface of pre-B cells and mature B cells, but is absent on stem cells and antibody producers - plasma cells. The administration of the drug rituximab, used for 10 years to treat lymphomas, leads to a rapid and deep (almost to zero) drop in the number of B cells in the peripheral blood, lasting up to 6 months or more.
- ACCP is an important marker of early rheumatoid arthritis
Studies have shown high efficacy and good tolerability of rituximab in patients with severe RA in whom previous therapy with MTX and TNF blockers was ineffective.
The 2007 consensus statement indicated that the advisability of prescribing rituximab can be considered not only after the ineffectiveness of previous treatment with TNF-α blockers, but also in the presence of contraindications to these drugs. Thus, it is recognized that rituximab may be the first among biological drugs prescribed to a patient with RA. In RA patients with concomitant B-cell lymphoma (including in the past), rituximab is the drug of choice.
Since September 2006, rituximab has been approved for the treatment of RA in Russia, and in April 2007, the Russian register of RA patients receiving this drug was created. This message is an analysis of the first results of the use of rituximab in Russia. We believe that our experience will be useful for rheumatologists who will work with this drug.
Material and methods.
To date, 42 patients with RA have received one full course of treatment with rituximab (2 infusions) (the social status of the patients is presented in Diagram 1). Among them there were 38 women and 4 men; the average age was 50.1±10.4 years, the average duration of the disease was 8.5±6.2 years. There were 39 patients (93%) seropositive for rheumatoid factor (RF). Radiological stage II of RA was diagnosed in 15 patients, stage III in 24 and stage IV in 3. Functional joint failure of stage I was present in 2 patients, stage II in 35 and stage III in 5. Systemic manifestations were noted in 35 patients (83%) ( diagram 2). The clinical characteristics of the patients are presented in Table 1. Previous therapy is shown in Diagram 3.
Diagram 1. Social status of patients
Diagram 2. Systemic manifestations of RA
Table 1. Clinical characteristics of patients
| Severity of pain (VAS*, mm.) | 68,0 ± 19,4 |
| Morning stiffness (min) | 220,3 ± 217,7 |
| Number of inflamed joints (out of 66) | 15,0 ± 12,2 |
| Number of painful joints (out of 68) | 20,3 ± 13,0 |
| ESR, mm/hour. | 38,66 ± 12,6 |
| SRP, mg/l | 28,2 ± 32,2 |
| Assessment of the severity of the condition (VAS, mm) by the patient | 66,1 ± 19,4 |
| doctor | 67,1 ± 19,5 |
| DAS28 | 6,6 ± 0,9 |
| Hemoglobin (g/l) | 113,1 ± 13,2 |
| Leukocytes (thousands in 1 cubic mm) | 8,6 ± 8,2 |
| Neutrophils (%) | 67,4 ± 15,3 |
| Platelets (thousands per 1 cubic mm) | 304,1 ± 95,8 |
Diagram 3. Previous therapy
- Some issues in the treatment of rheumatoid arthritis with concomitant chronic hepatitis B or C
Specific indications for rituximab were: ineffectiveness of infliximab in 4 patients, intolerance to it in 6, ineffectiveness of previous traditional basic therapy in 25 (including two or more basic drugs, including metho-rexate, in 22), intolerance to previous basic therapy in 5 , high laboratory activity in 1. One patient with early RA was prescribed rituximab as the first basic drug in order to achieve remission as quickly as possible.
The dynamics of the DAS28 indicator was chosen as the main indicator of the therapeutic effect of rituximab. The level of B cells (CD19+), RF, C-reactive protein (CRP), immunoglobulins (Ig) M, A and G, and standard hematological parameters were also taken into account separately.
The majority of patients (37) were administered rituximab according to the classical regimen: 2 intravenous infusions of 1000 mg each with an interval of 2 weeks. Before each administration, premedication was performed - 100 mg of methylprednisolone intravenously. Five patients received 2 infusions of rituximab, 500 mg each.
Results and discussion.
The administration of rituximab led to a sharp drop in the level of B cells in the peripheral blood in all patients in whom this indicator was assessed, to a very low level close to zero; Only single cells were detected in the preparations (Diagram 4).
Diagram 4. Dynamics of B-cell levels (CD 19) during treatment with rituximab
As a result of the therapy, a significant positive effect was observed, most clearly manifested in the dynamics of the number of inflamed (FWS) and painful (CBS) joints. These indicators significantly decreased by the 8th week. They then continued to decline steadily during the subsequent observation period and reached very low values by week 24 (Figure 5).
Diagram 5. Dynamics of the number of inflamed (PVS) and painful (PPS) joints
Laboratory indicators of inflammatory activity—ESR and CRP—also decreased noticeably (diagrams 6 and 7). Particularly noteworthy is the significant and relatively rapid decrease in the RF titer (Diagram 8), despite the lack of a significant decrease in the level of IgM (Diagram 9), which includes the standardly determined RF. This circumstance to some extent confirms the assumption of a more pronounced destruction of B cells that produce autoantibodies by rituximab. The content of IgA and peripheral blood parameters (diagram 10) did not change. At the same time, the level of IgG decreased significantly by the 16th week. Thus, an effect of rituximab on the number or function of plasma cells, despite the fact that they lack CD20 antigen, cannot be completely excluded.
Diagram 6. Dynamics of ESR
Diagram 7. Dynamics of SRP concentration
Diagram 8. Dynamics of rheumatoid factor
Diagram 9. Dynamics of immunoglobulin levels
Diagram 10. Dynamics of peripheral blood parameters
When individually assessing the effectiveness of therapy, a gradual increase in positive results was noted. Thus, according to the DAS28 criterion, good and satisfactory results after 8 weeks were recorded in 62% of patients, after 16 weeks - in 86%, and after 24 weeks - 100% (Diagram 11). The relatively slow and gradual development of clinical improvement with rituximab is consistent with the hypothesis that the main mechanism of action of this drug is inhibition of antigen-presenting B cell function.
Diagram 11. Evaluation of the effectiveness of therapy according to EULAR criteria after 8 (n=34), 16 (n=14) and 24 (n=8) weeks (%)
It is noteworthy that the effect of therapy did not show parallelism with the severity of the decrease in the level of B cells. Apparently, a decrease in their level in itself is not a sufficient condition for the development of clinical improvement in RA.
Rituximab was well tolerated. Basically, only infusion reactions were recorded in the form of facial swelling and nasal congestion in one patient, paresthesia of the upper respiratory tract in 2 patients, hyperemia and itching of the ears in 2, and flu-like syndrome in 2 patients. In one patient with stage I heart failure, after the 2nd infusion, increased shortness of breath and pain in the heart area were observed.
Thus, the use of rituximab for the treatment of RA not only opened a new direction in antirheumatic biological therapy, but also expanded our understanding of the pathogenesis of this disease, emphasizing the important role of B cells. This drug deserves widespread use in the treatment of severe RA patients refractory to previous therapy, including TNF-a blockers. Its tolerability turned out to be quite satisfactory.
The immediate tasks in the study of rituximab are to develop indications for its repeated courses and evaluate its anti-destructive effect.
April 1, 2008
Side effects
The use of drugs with monoclones is always accompanied by the development of side effects. To a lesser extent, negative reactions of the body are observed when taking the drug Stelara. This is due to the fact that the drug contains only human monoclones.
Otherwise, the side effects are the same for all drugs in this group. These include:
- disorders of the nervous system;
- symptoms of intoxication;
- signs of respiratory system disorders;
- local reactions in the area of drug administration;
- disorders of the urinary system;
- disruptions in the functioning of the cardiovascular system.
In most cases, there are negative reactions from the digestive tract - diarrhea and constipation, stomach pain, cramps, increased gas formation.
From the nervous system, disorientation, dizziness, migraine, anxiety, asthenic syndrome are possible. Patients often experience symptoms of intoxication during treatment with drugs containing monoclones. After administration of the drug, a violation of the immune system is possible, which can lead to exacerbation of chronic diseases, including infectious ones.
With individual intolerance, allergy symptoms appear - itchy skin, urticaria, swelling at the injection site and symptoms of intoxication.
Possible disruptions in the functioning of the cardiovascular system - sudden surges in blood pressure, tachycardia, arrhythmia. For this reason, the drugs are not recommended for older patients, especially in the presence of any vascular pathologies.
The urinary system may respond to the administration of the drug with dysuria. Patients often complain of difficulty urinating, nighttime urge to go to the toilet, spastic and girdling pain in the kidney area.
One of the possible side effects when taking the medicine is alopecia. Hair loss is considered a fairly common reaction of the body to treatment with monoclonal antibodies. As a rule, alopecia is temporary and hair is restored as the body gets used to the action of the drug.
Women also note increased brittleness of nails and dry facial skin. These phenomena are also short-term.
httpv://www.youtube.com/watch?v=embed/p8g-LIcgQU0




![Table 1. Main risk factors for AP and bone fractures [2]](https://shoes-clinic.ru/wp-content/uploads/tablica-1-osnovnye-faktory-riska-op-i-perelomov-kostej-2-330x140.jpg)