Competition "bio/mol/text"-2017
This work was published in the “Biomedicine Today and Tomorrow” category of the “bio/mol/text” competition 2017.
The general sponsor of the competition is: the largest supplier of equipment, reagents and consumables for biological research and production.
The sponsor of the audience award and partner of the “Biomedicine Today and Tomorrow” nomination was.
"Book" sponsor of the competition - "Alpina Non-Fiction"
Systemic rheumatic diseases are pathologies that arise due to the aggressive effects of the immune system on one’s own tissues. Their development is based on an error of the immune system, which incorrectly recognizes the normal components of the human body - autoantigens . Immune cells mistake them for foreign agents, which they see as a threat to the body. The protective function is activated, and healthy cells are “bombarded” by factors of the immune system - autoantibodies (Fig. 1).
The basics of normal immunity and the autoimmune process are presented in an accessible form on “Biomolecule” in the article “Immunity: the fight against strangers and... our own” [1].
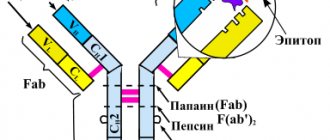
Figure 1. Diagram of the structure of antibodies. Antibodies are immunoglobulin proteins that have two H chains (heavy) and two L chains (light). Each protein molecule contains specific Fab fragments . These regions are responsible for binding to the antigen , the “target” that the antibody acts on. The structure of the Fab fragment is very variable, which allows it to adapt to the active centers of the antigen - epitopes . Chemical bonds (ionic, hydrogen, hydrophobic) are formed between the antibody and antigen. The other end of the molecule, the Fc fragment , is responsible for binding the resulting immune complexes to the Fc receptor located on the membranes of immune cells (neutrophils, macrophages, mast cells). Activation of immune components triggers a “killing reaction” against a foreign antigen. Antibody-dependent cytotoxicity occurs in this way.
“Antibodies, structure and functions of immunoglobulins”
The theory of autoimmunity was formulated a century ago by the German researcher Paul Ehrlich. Over the following years, many autoimmune diseases have been described. These include rheumatoid arthritis, systemic lupus erythematosus (SLE), systemic scleroderma, myopathies, vasculitis and other pathologies.
You can read more about the mechanisms of development of some rheumatic diseases in the articles: “Systemic lupus erythematosus: a disease with a thousand faces” [2] and “Rheumatoid arthritis: change the composition of joints” [3].
Diseases associated with an autoimmune component are a serious problem in modern society. Their prevalence in the world population is approximately 5%. Diseases quickly become chronic, which reduces the quality of life of patients. Autoimmune pathologies often lead to disability in patients [1], [4].
In 2021, Biomolecule published a special project dedicated to autoimmune diseases.
Despite many years of searching for new ways of pharmacotherapy, modern medicine cannot offer treatment methods that directly affect the cause of autoimmunity. Doctors can only slow down the progression of the pathology and reduce the severity of the clinic - carry out pathogenetic and symptomatic treatment. For this purpose, basic therapy has been developed and has been used for many years. However, proven drugs do not always work as they should.
Why is it necessary to look for new treatments?
A wide range of antirheumatic drugs are used to treat autoimmune diseases. Classical therapy includes nonsteroidal anti-inflammatory drugs, glucocorticoids, and cytostatics. Depending on the characteristics of the development of the disease, drugs from one or another group are selected [4].
Medicines used for rheumatic diseases
- Major immunosuppressants:
- glucocorticoids (prednisolone, dexamethasone);
- antibiotics (cyclosporine, tacrolimus);
- antimetabolites (methotrexate, azathioprine, mercaptopurine);
- cytostatics (cyclophosphamide).
- Minor immunosuppressants:
- Plaquenil, hingamin;
- cuprenil;
- gold preparations (auronofin);
- heparin;
- non-steroidal anti-inflammatory drugs.
Let's figure out why new drugs are needed. As an example, we can consider the classical therapy of one of the most common autoimmune diseases - rheumatoid arthritis [3]. Modern strategies to combat this pathology must comply with the concept of Treat to target - “treatment until the goal is achieved.” It is aimed at remission (disappearance of symptoms) of the disease or a sharp decrease in the activity of arthritis [5], [6].
The “gold standard” in treating the disease is methotrexate (Fig. 2). The drug belongs to the group of basic anti-inflammatory drugs.
Figure 2. Structural formulas of methotrexate and folic acid. The drug ( a ) is similar in structure to folic acid ( b ). It consists of pteridine groups and para-aminobenzoic acid. The active component of methotrexate differs from the structure of folate in the absence of a hydroxyl group (–OH) and the presence of an additional methyl radical (–CH3).
"Wikipedia"
According to the mechanism of action, methotrexate is an antimetabolite. Drugs in this group inhibit physiological reactions in the body by binding to enzymes and turning off their activity. A substance that normally participates in the reaction can no longer enter the biochemical cycle due to increased competition with the components of the drug. The main target for the action of methotrexate is the enzyme that breaks down folic acid, dihydrofolate reductase ( DHF ). This protein acts on the folate entering the body, converting it into its active form. This produces tetrahydrofolic acid. They are involved in the synthesis of building materials for DNA - purine bases and thymidylate.
Methotrexate enters the chain of reactions due to its structural similarity to the folic acid molecule (Fig. 2). Competition for the enzyme leads to the fact that the content of active folate in the tissue is significantly reduced. There is no building material - there are no new DNA molecules, without which cells cannot reproduce. Those tissues whose structural elements are constantly dividing have increased sensitivity to the effects of the drug. These also include bone marrow components from which future immune cells are formed.
Another important point in the work of methotrexate is related to the activity of its polyglutamated metabolites. These molecules are formed after activation of the drug directly in the cells of the human body. They inhibit other enzymes that interact with folic acid. This group includes thymidyl synthetase and AICAR transamylase. The activity of methotrexate derivatives triggers the production of adenosine. It has a powerful anti-inflammatory effect, which reduces the severity of symptoms of rheumatoid arthritis [7].
Methotrexate is convenient to use - it is easily dosed and can be prescribed in long courses. In addition, an important advantage of this drug is its low price, in comparison with modern drugs from imported pharmaceutical companies [8]. Despite all the advantages, treatment with methotrexate does not always lead to a decrease in disease activity. In many patients, the use of the drug is ineffective even in combination with other classical drugs [5]. This is confirmed by analyzing statistics. Studies have shown that when treated with methotrexate and combinations of disease-modifying drugs, only half of patients achieved remission [9], [10].
The failure of classical therapy forces scientists to look for new ways to treat rheumatoid arthritis. It is worth noting that, despite new developments, the fight against the disease still begins with the prescription of methotrexate and its analogues. Additional drugs are used only when the autoimmune process is highly active. In such patients, the use of one drug is often ineffective [7]. Depending on the characteristics of the pathology, a suitable treatment regimen is selected (Fig. 3).
Figure 3. Management tactics for a patient with rheumatoid arthritis when methotrexate is ineffective.
“Pharmacotherapy of rheumatoid arthritis from the perspective of evidence-based medicine: new recommendations”
The ineffectiveness of basic therapy drugs occurs not only in rheumatoid arthritis, but also in other diseases of autoimmune origin (systemic lupus erythematosus, scleroderma, ankylosing spondylitis). This motivates rheumatology specialists to develop other drugs and select new treatment regimens.
Choosing a basic drug in the treatment of rheumatoid arthritis
Rheumatoid arthritis (RA) is a severe disabling disease, for the treatment of which it is necessary to prescribe basic drugs as early as possible. Of the drugs used as baseline drugs, the most widely used today are methotrexate (the gold standard for the treatment of RA) and sulfasalazine. Unfortunately, the number and severity of side effects caused by the use of these drugs sometimes exceed the severity of the therapeutic effect. In this regard, the release of new, safer drugs for the treatment of rheumatoid arthritis (RA) is of particular importance at the moment.
The only new first-line drug for the treatment of this pathology, introduced into clinical practice over the past 10 years, is Arava (leflunomide) produced by pharmaceuticals (France). Possessing an original mechanism of action, Arava significantly improves the clinical course of the disease, the functional state of patients and sharply slows down the radiological progression of RA. The results of clinical studies that examined the relationship between the effectiveness and safety of the drug were discussed at a conference dedicated to the prospects for the treatment of rheumatoid arthritis.
Doctor of Medical Sciences, Professor of the Department of Hospital Therapy No. 1 of NMU Oleg Borisovich Yaremenko gave a review of new data on the study of the effectiveness of Arava in the treatment of RA and the safety of its use.
— During the use of Arava in the world (more than five years), hundreds of thousands of RA patients have accumulated vast experience, allowing us to draw informed conclusions about the role of this drug in the treatment of rheumatoid arthritis. The key point in assessing the need for the use of Arava in basic therapy is the benefit-risk ratio of the therapy.
Numerous long-term studies were conducted in this direction, based on the results of which at the end of 2002 the FDA (USA) commission conducted a general analysis. The following sources of information for assessing the effectiveness and safety of Arava were considered:
- clinical trials database;
- post-marketing studies of combination therapy;
- the Aetna database (the largest adverse event database to date);
- analysis of Protocare/Pharmetrics studies;
- national data bank on RA therapy;
- data from the Acute Liver Failure Case Study Group (USA);
- analytical database of side effects.
The results of not only clinical, but also post-marketing studies have clearly shown that Arava is a highly effective drug in the treatment of RA. One of the most significant advantages of the drug is the early onset of clinical action, on average 4 weeks after the start of use. In addition, Arava, to a greater extent than other basic drugs, improves the functional state of patients, and this is reflected in the recommendations of the FDA commission, which recorded new indications for prescribing leflunomide for RA, namely, improving the physical condition of patients (increasing functional activity).
Side effects were also considered by this commission, focusing on the issue of hepatotoxicity, which can significantly limit the use of one or another basic drug.
First of all, the frequency of side effects of Arava and traditional drugs for the basic therapy of RA - methotrexate and sulfasalazine - was compared. When compared with methotrexate, it turned out that the incidence of side effects, both general and those involving increased levels of liver enzymes (ALT and AST), was higher with methotrexate. When compared with sulfasalazine, the incidence of adverse liver reactions was approximately the same.
According to the Aetna database, which contains information on more than 40 thousand medical records, the frequency of hepatic side effects among Arava, methotrexate and other disease-modifying drugs, as well as combination disease-modifying therapy (Arava + methotrexate, Arava + DMARD, methotrexate + DMARD) did not differ significantly or was less when using Arava. Complications such as liver necrosis, hepatic coma, non-infectious hepatitis, parenchymal jaundice, liver cirrhosis, increased liver enzymes and other pathological changes were taken into account.
It should be noted that the incidence of severe conditions (acute liver necrosis, non-infectious hepatitis, parenchymal jaundice and cirrhosis) was lower when taking Arava than when using methotrexate and other basic drugs.
Due to the fact that liver transplantation is quite widely practiced in the United States, it is important to study the effect of the use of Arava on the frequency of such operations. It was found that the incidence of liver transplantation among RA patients taking Arava was 2 times lower than when using other basic drugs.
Thus, after analyzing the frequency of side effects, in particular liver complications, the FDA commission came to the following conclusions:
- increased levels of liver enzymes are a common side effect of basic RA therapy and amount to 2-4%;
- serious side effects of therapy requiring hospitalization are rare, their frequency is about 0.01-0.02%;
- the frequency of side effects with Arava therapy is at least no higher than that with other basic drugs for the treatment of RA;
- the ratio of the benefits of Arava therapy to the risks of the therapy is favorable.
Thus, Arava is a highly effective basic drug for the treatment of RA; in comparison with methotrexate and sulfasalazine, it is less likely to cause side effects, which indicates a positive benefit-risk ratio and allows Arava to be widely used as a basic drug for RA.
Just six months after the above meta-analysis, many new publications, reviews and meta-analyses, as well as the results of randomized trials, have appeared that confirm or complement the existing information.
In particular, a fairly large study was completed in China (C. Bao, S. Chen, Y. Gu et al.), in which about 500 patients took part. It compared the effectiveness of Arava and methotrexate. It turned out that according to the criteria of the American College of Rheumatology (ACR 20), the number of positive clinical responses to treatment with Arava was slightly higher than to therapy with methotrexate (62.5% compared with 60.1% after three months; 67.2% compared with 61.3% six months after the start of treatment). In this study, the incidence of serious adverse events requiring drug discontinuation was significantly lower with Arava (16.8% compared with 28.2% with methotrexate).
A rather original approach to assessing the effectiveness and tolerability of basic therapy for RA was used by English scientists (F. Wolfe, B. Stephenson, J.-J. Doyle), who chose the following main criteria: the number of cases of changes in the basic therapy regimen (or drug) and the duration of treatment drug. The study lasted 3 years; Arava and methotrexate were used as basic therapy in RA patients.
The therapy changes were as follows:
- prescribing another drug as an additional one;
- discontinuation of the drug, prescription of another.
The number of cases of changes in basic therapy was 55.5% for Arava and 57.3% for methotrexate, the duration of effective treatment before changing therapy was 15 months for Arava and 14 months for methotrexate.
The press published data from a meta-analysis of controlled clinical trials of the effectiveness and safety of Arava in the treatment of RA (M. Osiri, V. Robinson et al.), which covered 6 clinical studies that included 2044 RA patients. The data obtained confirmed the existing information about the effectiveness and safety of Arava therapy.
In particular, the meta-analysis emphasized:
- 2 times more patients responded to treatment with Arava after 6 and 12 months according to the ACR 20 criterion compared to the placebo group;
- the effect of treatment with Arava develops quickly - within 4 weeks;
- Arava improves all clinical parameters and slows the radiographic progression of RA at 6 and 12 months of treatment;
- the number of patients dropping out during treatment with Arava is lower compared to the placebo group. In terms of side effects during two years of treatment, Arava is comparable to sulfasalazine and methotrexate.
In 2001, for the first time at the American Congress of Rheumatology, a group of Canadian researchers reported that, according to their data, the effectiveness of Arava in doses of 10 mg and 20 mg was not significantly different, and the number of side effects when using a dosage of 20 mg was slightly higher than when using a 10 mg dosage. To confirm these data, a double-blind randomized study was conducted. It involved 400 patients (83.3% of them women, average age 55.5 years) from 70 centers. The average duration of the disease was 9.6 years, in 92.5% of cases - functional class according to ACR II / III. The double-blind phase lasted 24 weeks.
This study found that the number of patients who responded to treatment according to ACR criteria was approximately the same when using Arava at doses of 10 and 20 mg, both according to ACR 20 criteria (50 and 57%, respectively) and ACR 50 criteria ( 20 and 26%, respectively), as well as ACR 70 (7 and 10%, respectively).
When studying various dosages of Arava, another very important criterion from a clinical point of view was taken into account - the effect of the dosage of Arava on the dose of glucocorticoids. For this purpose, the study included only those patients who started taking glucocorticoids before Arava was prescribed. The following results were obtained: glucocorticoids were canceled in 2% of patients when using 10 mg of Arava and in 7% when using 20 mg; the dose was reduced in 17 and 19% of patients, respectively; an increase in the dose of glucocorticoids was observed in 22 and 7%, respectively.
As for the side effects of Arava at different dosages, no statistically significant differences could be identified, except for an increased incidence of diarrhea and nausea when using Arava at a dose of 20 mg.
This meta-analysis allows us to draw conclusions.
- The effectiveness of Arava doses of 10 and 20 mg is almost equal.
- The use of Arava at a dose of 20 mg allows, in contrast to a dose of 10 mg, to reduce the dose of glucocorticoids.
- Arava at a dose of 20 mg is more appropriate to use in patients with higher activity of the process, that is, with the simultaneous use of glucocorticoids.
- Data on the efficacy and safety profile of Arava confirm information obtained in previous international clinical studies.
Judging by the accumulated data, the greatest experience in using Arava in Ukraine is in the clinic of the Department of Therapy, Faculty of Medicine No. 2, Vinnitsa National Medical University. N. I. Pirogov (N. A. Stanislavchuk and S. V. Shevchuk). This clinic conducted an interesting study in which the effectiveness of treatment with methotrexate (9.2 mg/week), Arava (20 mg/day) and Arava (10 mg/day) in combination with methotrexate (7.5 mg) was assessed in three groups of patients. /week). It should be emphasized that this is the fourth study in the world to study the combined effects of Arava and methotrexate and the only study in which such small doses of Arava and methotrexate were used. All previous studies examined the effect of Arava at a dose of 20 mg, methotrexate at a dose of 15 mg.
According to the authors, one month after the start of therapy, the response to treatment according to the ACR 20 criterion was on average 2 times higher in the groups of patients receiving Arava than when using methotrexate alone. There were no significant differences in response to Arava monotherapy and its combination with methotrexate. 2 months after the start of the study, the best response to treatment was obtained when using Arava in combination with methotrexate, the least pronounced effect was observed with methotrexate monotherapy. After 6 months, the effectiveness rates for combined treatment (Arava + methotrexate) increased sharply and were one and a half times higher than those for monotherapy with Arava or methotrexate, the therapeutic response to which remained virtually unchanged.
Approximately the same pattern was observed when using the DAS criterion recommended by the European League Against Rheumatism.
Side effects were most pronounced in the group of patients receiving combination treatment (Arava + methotrexate); the rate of treatment discontinuation in this group due to side effects was 19.5%, which was 2 times higher than the same indicators for methotrexate monotherapy (8.2% ) and Arava (10%). Side effects were mainly diarrhea and elevated liver enzymes.
In addition, the drug was found to have a number of additional clinical and laboratory effects, which allows us to consider expanding the indications for the use of Arava.
Even at the preclinical stage of the study, a significant decrease in the level of uric acid and phosphates in the blood was revealed when using leflunomide. Reducing phosphate levels remains without practical application, since its clinical significance is unclear. Reducing uric acid levels is of great interest. It was to test this phenomenon in a clinical setting that this year Spanish authors (F. Perez-Ruiz, JM-J. Nolla) conducted a study, the results of which indeed confirmed a significant decrease in uric acid levels when using Arava and some time after its discontinuation.
These properties allow the use of Arava in patients with rheumatoid arthritis with concomitant hyperuricemia or gout.
Attempts have been made to use this drug to treat inflammatory joint diseases, as well as systemic connective tissue diseases. It should be considered advisable to prescribe Arava as an adjuvant for spondyloarthropathies (psoriatic arthritis, ankylosing spondylitis), SLE and vasculitis.
The most convincing evidence comes from psoriatic arthritis, which rheumatologists often call the “little brother” of rheumatoid arthritis. Unfortunately, the problem of basic therapy for psoriatic arthritis is even more complex than for rheumatoid arthritis. The effect of the main drugs (sulfasalazine, methotrexate, cyclosporine, azathioprine, gold preparations) is extremely low in the presence of articular syndrome in the psoriasis clinic.
To date, only two open studies have been conducted to study the effectiveness of Arava in the treatment of psoriatic arthritis (Liang and Scarpa). Their results are as follows:
- Liang: improvement in all criteria after using Arava at a dose of 10-30 mg per day for 23 months.
- Scarpa: improvement in arthritis but no effect on skin after 3 months.
A randomized, blind study (Treatment with Leflunomide in Psoriatic Arthritis, TOPAS) was also completed - a multicenter, double-blind, placebo-controlled study in which Arava was used to treat patients with psoriatic arthritis for 6 months according to the following regimen: 100 mg per day for 1- 3 days, then 20 mg per day. The results of this study showed that in 188 patients who received Arava, improvement in PsARC response criteria was observed in almost 60% of cases, which is 2 times higher than in the placebo group. According to the modified ACR criteria, the same pattern was revealed: Arava was effective in 36.3% compared to 20% when taking placebo.
Positive changes in articular syndrome with the use of Arava were reflected in the physical condition of patients, which improved 4 times compared to placebo.
Almost half of the patients experienced improvement in a number of other (non-articular) manifestations of psoriasis, which was 2 times greater than the placebo effect. In particular, there was a positive effect on the condition of the skin and mucous membranes, which none of the basic drugs has provided so far.
Side effects were consistent with those previously reported, with a slightly higher incidence of diarrhea. This may be explained by the more stringent diagnostic criteria in the study, since a higher rate was also observed in the placebo group.
The TOPAS study led to the following conclusions:
- Arava demonstrated superior efficacy compared to placebo in the treatment of patients with psoriatic arthritis;
- when treated with Arava, both joint and skin manifestations of the disease improve;
- Arava has an acceptable safety profile and is generally well tolerated by patients.
Numerous studies on the experience of using Arava in clinical practice have shown its high effectiveness, comparable to the effectiveness of traditional basic drugs, as well as acceptable safety indicators for its use, which allows us to recommend the drug for the basic treatment of rheumatoid arthritis, as well as other diseases, in particular psoriatic arthritis. The drug enjoys well-deserved attention from practitioners all over the world, which was once again proven by an interactive survey of rheumatologists organized at the EULAR Congress in 2003 (in Lisbon). According to this survey, 74% of rheumatologists use Arava (leflunomide) for combination therapy for rheumatoid arthritis and 57% for the treatment of psoriatic arthritis.
The data presented in the review report by Oleg Borisovich Yaremenko was confirmed and supplemented by the experience of using leflunomide by Ukrainian clinicians at the Institute of Cardiology named after. N.D. Strazhesko, who was presented by an employee of the institute, Elena Alekseevna Garmish.
— The purpose of the study conducted at the Institute was to evaluate the effectiveness and safety of the use of leflunomide for 12 months in patients with rheumatoid arthritis.
To assess the effectiveness of treatment, we used: ACR remission criteria; articular (SI), pain (BI) and inflammatory (VI) Ritchie indices; the duration of morning stiffness was determined; the patient's functional ability according to the Lee, HAQ indices; number of erosions according to radiography and MRI; changes in the synovium according to MRI.
The study included 19 patients, average age 45.4 years, with the following characteristics of the course of RA:
- The duration of the disease was more than 2 years in 14 patients (group II) (on average 5.5 years); in only 5 patients (group I) - less than 2 years (average 6.8 months);
- X-ray stage I of the disease - in 5 patients; II - in 8 and III - in 7 patients;
- I degree of disease activity was present in 3 patients; II - in 9 and III - in 7 patients;
- All patients took NSAIDs according to the standard regimen; 90% of patients needed corticosteroids.
Leflunomide (Arava) was used at a dose of 100 mg per day for the first 3 days, then 20 mg per day for a year.
According to ACR criteria, convincing data on the effectiveness of Arava were obtained: according to ACR 20 criteria, the proportion of responders to therapy increased from 57% after the first month of using the drug to 72% at the end of the year; according to ACR 50 criteria - from 31 to 55%, respectively; response to therapy according to ACR 70 criteria appeared in the 3rd month and increased from 5 to 27% by the end of the year.
When assessing the dynamics of the number of swollen and painful joints, the following data were obtained: the number of painful joints decreased by an average of 69%, swollen joints by 76%, while the response in group I patients (that is, with a shorter duration of the disease) was much more pronounced.
Due to the decrease in pain and swelling of the joints, their functional activity increased, it improved by an average of 45%, which was more pronounced in group I patients.
The positive effect of Arava was also noted in relation to the dynamics of pain and RA activity according to VAS, as well as when analyzing other indicators. In particular, the Ritchie indices, which reflect the increase in the rate of effect of the drug, show that by the end of the first month of taking Arava, its effectiveness is about 40%, which gradually increases and is maintained until the 12th month. In accordance with this, the Lee index, which reflects the patient's disability, decreases.
The effectiveness of Arava was assessed by the number of bone erosions. Before Arava’s appointment, according to X-ray data in group I, only one case of erosion was noted (in the wrist area), according to MRI - 20 erosions, most of them in the bones of the wrist. In group II, 24 erosions were identified using radiography, and 113 erosions using MRI in all anatomical areas (the predominant number were in the bones of the wrist). Joint effusion was also detected in 8 patients and tenosynovitis in 3 patients.
The results of instrumental research methods after 12 months of using Arava showed:
- according to X-ray examination, no increase in the number of erosions was registered;
- according to MRI data, in 6 out of 10 patients the dynamics of erosion stopped, in 4 the contour of erosion changed (became clearer, which was regarded as stabilization of the process);
- the thickness of the synovial membrane decreased from 5±1.9 to 2.47±0.7 mm;
- Joint effusion and tenosynovitis were absent in all patients.
The effect of Arava on the need for NSAIDs and corticosteroids deserves special attention. After treatment, 55% did not need to take NSAIDs, 44% of patients did not need corticosteroids; By the 12th month of using Arava, 5 patients in group I and 3 patients in group II were completely stopped taking NSAIDs and corticosteroids; in the remaining patients, the dose of corticosteroids was reduced.
We continue to monitor 7 patients who have been continuously taking Arava for 2 years. They do not need to use corticosteroids; they occasionally take NSAIDs. The effectiveness of Arava in all patients also does not decrease in comparison with what was achieved by the 12th month of the study.
The drug was discontinued in only one patient due to exacerbation of bronchial asthma (but we do not associate this directly with the effect of this drug). Among the side effects, gastrointestinal disorders were observed in the form of increased motility, some loosening of stools and nausea (2 patients), hair loss (2 patients), leukopenia (1 patient). Therefore, in 2 patients (with leukopenia and gastrointestinal pathology), the dose of Arava was reduced to 10 mg per day.
An interesting case of a decrease in proteinuria in one of the patients who was prescribed Arava with proteinuria of 9.9 g/l, which developed as a result of taking another drug. By the end of the year, the patient's proteinuria level decreased to 0.66 g/l, and a pronounced anti-inflammatory effect was observed.
Based on these data, we can conclude that Arava (leflunomide) is a highly effective drug in the treatment of rheumatoid arthritis at all stages of the disease, the therapeutic effect of which is stable throughout the entire treatment period. Considering the relationship between the effectiveness and safety of leflunomide, Arava can be recommended for the basic treatment of rheumatoid arthritis, both in monotherapy and in combination with methotrexate or sulfasalazine.
Source of information: https://www.esus.ru
The article was published on the website https://www.rusmg.ru
Genetically engineered drugs: addition to basic therapy
monoclonal antibodies has provided new insight into the treatment of autoimmune diseases [11]. A fundamentally new class of drugs has been obtained thanks to the achievements of genetic engineering. To understand the mechanism of action of these drugs, it is worth remembering how immune cells work in health and disease [1], [12].
The immune system is a complex mechanism consisting of many “cogs” - immune cells. Each of them has its own functions and occupies a certain place in the overall structure of the protective system. In response to the arrival of an “enemy” agent (antigen), components of the innate immune system—nonspecific protective factors—are activated. These are neutrophils, eosinophils and basophils, which are the first to stand in the way of harmful effects.
The cogs turn and new components of the immune system are activated. T- and B-lymphocytes enter the fight against the pathogen. They include more subtle defense mechanisms - specific cytotoxicity. Antibodies are produced, T-killers look for their “victim”... Fine regulation of the process with the help of cytokines allows you to quickly achieve your goal. The coordinated action of all components of immunity leads to the implementation of the program - the destruction of the pathological agent.
During the selection of suitable “parts” for the mechanism - during the selection of lymphocytes - errors inevitably arise. The immune system produces autoreactive clones—cells that are specific to body tissue antigens. Normally, they are eliminated in the “workshops” - the thymus and lymph nodes. Those clones of lymphocytes that do not distinguish between self and foreign antigens are immediately destroyed even before they begin to perform their function. But what happens when the cogs fall out of the immune machine? The breakdown occurs in a specific part of the mechanism - in the work of T- and B-lymphocytes. If the selection process is disrupted, autoreactive cells are released into the blood. They look for their “victims” and find them in the normal elements of their own tissues.
Depending on the type of reaction, the pathophysiological process underlying autoimmune aggression differs. T-lymphocytes can kill body cells on their own, or they can work “by proxy” - activating the production of autoantibodies by B-lymphocytes. When B-cell immunity is damaged, autophagy is realized through the complement system, as well as through the formation of cytotoxic immune complexes [13], [14]. You can read more about the mechanisms of normal and altered immune responses on Biomolecule [1], as well as in articles [15], [16].
In autoimmune diseases, it is possible to suppress the entire complex mechanism of immunity at once, which is what classical therapy drugs do. But this leaves a person without protection from enemy agents - bacterial infections, viruses and other pathogens. Therefore, it is preferable to preserve the activity of the immune system as a whole, saving a person from auto-aggression of certain of its components. This is exactly how new drugs—monoclonal antibodies—work.
Biological agents affect individual “cogs” of the immune defense mechanism. Their targets may be cytokines and their receptors, membrane molecules of lymphocytes. Depending on the point of application of the drug, monoclonal antibodies are divided into groups (Fig. 4):
- TNF (tumor necrosis factor) inhibitors - infliximab, etanercept, certolizumab, golimumab, adalimumab.
- Interleukin receptor blockers - tocilizumab (IL-6R), canakinumab (IL-1R), secukinumab (IL-17R).
- Anti-B-cell antibodies (antibodies to membrane molecules CD20) - rituximab, belimumab [17].
- Anti-T-cell antibodies (antibodies to CD80 and CD86 molecules) - abatacept [18].
Figure 4. Pathophysiological “victims” of monoclonal antibodies - interleukins, TNF, surface proteins of lymphocytes.
“Individualization of treatment for rheumatoid arthritis: a course to achieve optimal results”
TNF inhibitors
Tumor necrosis factor inhibitors are the first monoclonal antibodies introduced into rheumatological practice. This group includes infliximab, etanercept, certolizumab, golimumab, adalimumab.
Tumor necrosis factor ( TNF ) is a pro-inflammatory cytokine (a substance that stimulates the development of an inflammatory response). Normally, when it is released, vascular cell proliferation, macrophage activation, and lysis of tumor agents occur. These effects play an important role in protecting the body from pathogens. Inflammation can be considered a response to damaging factors.
However, the effect of TNF on joints in rheumatic diseases cannot be called positive. Thus, in rheumatoid arthritis, the cytokine stimulates the proliferation of synovial fibroblasts - cells of the joint lining. This leads to the formation of pannus - growths of aggressive tissue. As the disease progresses, the process of inflammation and destruction spreads to the articular cartilage and underlying bones (Fig. 5). The joint tissues are filled with immune cells - macrophages, T- and B-lymphocytes, neutrophils. These mechanisms underlie the development of chronic inflammation. You can refresh your knowledge about the pathogenesis of rheumatoid arthritis in the article “Rheumatoid arthritis: change the composition of the joints” [3].
Figure 5. Pathological changes in the joint in rheumatoid arthritis. The autoimmune process causes erosions, synovitis (inflammation of the synovial membrane), and destruction of articular cartilage.
website krovetvorenie.ru
One of the TNF inhibitors is the drug infliximab. It has "human" and "mouse" areas. Approximately 25% of all amino acids in the monoclonal antibody are derived from mice. This is a Fab fragment - a specific region responsible for binding to TNF. The Fc fragment of the protein is formed from IgG1, a human antibody.
This structure is associated with the mechanism for obtaining the drug. Initially, an antibody to tumor necrosis factor is synthesized in the mouse. The resulting immunoglobulin is specific to TNF and can already neutralize it, but completely foreign proteins, of course, cannot be introduced into the patient’s body. This will cause an active immune reaction - the production of antibodies against therapeutic agents. Therefore, mouse immunoglobulin domains are replaced with similar regions of human proteins. Antibodies that have fragments of different origins are called chimeric. In fact, they take the best qualities of their predecessors. The mouse part provides high sensitivity to TNF, and the human fragments reduce immunogenicity - the likelihood of developing an immune response.
The mechanism of action of infliximab is clear from its structure. The Fab fragment of the molecule binds tumor necrosis factor, forming a stable complex with it. This interaction completely blocks the activity of the cytokine, preventing its connection with the membrane receptors p55 and p57. Infliximab “neutralizes” both soluble and membrane-associated forms of TNF (Fig. 6). The content of other pro-inflammatory factors, such as IL-1, IL-6, and nitrogen monoxide, also decreases in joint cells.
Figure 6. Main effects of TNF and monoclonal antibodies blocking it (infliximab and etanercept). The targets for monoclonal antibodies are free and membrane-associated forms of tumor necrosis factor. The drugs prevent the cytokine from binding to the receptor, thereby reducing the activity of rheumatoid arthritis.
website sportwiki.to
Another effective drug from the group of TNF inhibitors, etanercept, . It contains the extracellular part of the receptor for tumor necrosis factor. It “connects” to human IgG1. The hybrid molecule enters into fierce competition for free TNF and neutralizes it before the cytokine has time to contact receptors and trigger an inflammatory response. An additional effect of etanercept that other TNF inhibitors do not have is the neutralization of lymphotoxin. This substance also belongs to pro-inflammatory cytokines. The production of lymphotoxin stimulates proliferative processes in the joints. Accordingly, blocking its action reduces the activity of inflammation in rheumatological diseases [18].
TNF inhibitors have shown good results not only in the treatment of rheumatoid arthritis, but also in other autoimmune pathologies. For example, new drugs are widely used in patients with ankylosing spondylitis. Slowing the progression of pathology in this case is very important, since auto-aggression is aimed at the joint and bone formations of the spine. Over time, the disease turns the spinal column into a “bamboo stick” - a monolithic, rigid formation. Ankylosis develops gradually, but inevitably. Every year the motor abilities of patients become more and more limited. The use of biological drugs can reduce the activity of inflammation in the spine. This slows down the process of ankylosis formation [19].
Interleukin receptor blockers
Interleukins play an important role in the development of autoimmune inflammation , which, like TNF, are proinflammatory cytokines (Fig. 7). The main representatives of this group are IL-6, IL-1, IL-17. The function of interleukins is the control of the processes of differentiation, proliferation and death (apoptosis) of immune cells, which is carried out through the corresponding target genes [20].
Figure 7. The mechanism of action of interleukins in autoimmune inflammation (using the example of IL-6). The cytokine affects T- and B-lymphocytes, hematopoietic cells, and hepatocytes. It stimulates the production of autoantibodies by B cells, as well as the formation of autoreactive T clones, which are directly involved in the autoimmune process. The effect on the bone marrow is to stimulate the production of new blood cells - the number of leukocytes and platelets increases. Inflammation is accompanied by a response of liver cells, the appearance of characteristic symptoms of an autoimmune disease.
[21]
The influence of interleukins is one of the “triggers” of the inflammatory process. Therefore, blocking their activity improves the condition of patients with autoimmune diseases. You can stop the work of interleukins by binding their receptors - molecules that transmit a signal to immune cells. This is the basis of the mechanism of action of monoclonal antibodies from the group of interleukin receptor inhibitors.
Tocilizumab is a drug that blocks IL-6. The receptor for this substance consists of two components: membrane IL-6R (α chain) and glycoprotein g130 (β chain). The membrane part of the receptor binds to IL-6, forming a stable complex. Together they activate the g130 component, causing a change in its structure (homodimerization). A receptor complex of two g130 molecules is formed, which in turn activates JAK1 kinase. This enzyme triggers a cascade of reactions in the cell, which leads to the appearance of the biological effect of the cytokine - the development of inflammation. In some cases, IL-6 binds not the membrane, but the soluble form of the α-chain (Fig. 8). The mechanism of action of the receptor does not change in this case [21].
Tocilizumab works by competitive inhibition. Signaling molecules actively bind to the monoclonal antibody. The vacant place is filled - interleukin cannot form a complex with the receptor, which means it is not able to activate the inflammation process.
Figure 8. Mechanism of action of tocilizumab. The drug binds soluble and membrane IL-6 receptors, blocking signal transmission.
"Modern targets for targeted therapy of rheumatoid arthritis: from monoclonal antibodies to signal molecule blockers"
Tocilizumab is considered one of the safest drugs in the group of monoclonal antibodies. This allows it to be used for juvenile idiopathic arthritis, which occurs before the age of 16 years. Children react particularly acutely to toxic effects, so the drugs used in their treatment should have a minimum number of adverse reactions. The use of tocilizumab allows one to achieve the desired treatment effect without causing severe complications.
Anti-B cell therapy
One of the main elements involved in autoimmune inflammation are B lymphocytes . They produce autoantibodies that bind to healthy cells in the body. The resulting complex of antibody and autoantigen attacks the complement system or cytotoxic lymphocytes. This process underlies the inflammatory response in rheumatic diseases such as systemic lupus erythematosus. A separate article on “Biomolecule” is devoted to it: “Systemic lupus erythematosus: a disease with a thousand faces” [2].
Anti-B cell therapy drugs ( rituximab and belimumab ) block the activity of B cells by binding to their membrane CD20 molecules. Only certain categories of B cells have these substances. They are specific for pre-B lymphocytes and mature B lymphocytes. CD20 is not present in stem elements and pro-B cells, from which new lymphocytic elements will be formed. Membrane molecules of this type are not found in plasma cells that produce immunoglobulins [22].
Thanks to this feature, the CD20 protein is an ideal “victim” for biological drugs. When its activity is “turned off,” neither the formation of new lymphocytes nor the production of normal antibodies is disrupted. One drug with this mechanism of action is rituximab. The monoclonal antibody binds to the CD20 molecule. This leads to the launch of immunological reactions in relation to B-lymphocytes, which ensure the destruction (lysis) of these cells (Fig. 9).
Figure 9. Mechanism of action of rituximab. The Fab fragment of the monoclonal antibody binds to CD20 on the surface of the B lymphocyte. This triggers cell lysis, which can occur in several ways: through the complement system, the apoptosis program, or the aggression of natural killer cells and macrophages.
"Modern targets for targeted therapy of rheumatoid arthritis: from monoclonal antibodies to signal molecule blockers"
Anti-T cell therapy
Blocking the action of T-lymphocytes is possible due to the peculiarities of their activation. In order for a T lymphocyte to enter the autoimmune process and bind to an antigen, it must receive two signals from antigen presenting cells (APCs). The first signal ensures recognition of a specific autoantigen by T-cell receptors. The second signal is a nonspecific process of binding of membrane molecules CD80 and CD86 on the surface of APC with the CD28 receptor of the lymphocyte. The combination of these interactions causes activation of T cells, which in turn stimulate the production of proinflammatory cytokines. This is the main contribution of T lymphocytes to the autoimmune process.
Knowledge of the mechanism of T cell activation was used in the development of monoclonal antibodies. The main representative of anti-T-cell agents is abatacept . The drug is a protein consisting of two parts. The specific part is formed by the CTLA-4 molecule (cytotoxic lymphocyte antigen 4). The nonspecific region is the Fc fragment of human immunoglobulin G1 [23].
The effect of abatacept is aimed precisely at a nonspecific (costimulatory) signal. The CTLA-4 component binds the CD80 and CD86 proteins on the surface of antigen-presenting cells. The CD28 lymphocyte receptor can no longer interact with them, which is why the activation of the T cell is not completed (Fig. 10).
Figure 10. Mechanism of action of abatacept. Abatacept modulates the immune response through binding to CD80/CD86 on antigen presenting cells. This prevents CD80/CD86 from binding to CD28 T cells, i.e. T cell activation is abolished through blocking costimulation.
"Modern targets for targeted therapy of rheumatoid arthritis: from monoclonal antibodies to signal molecule blockers"
Use of Sulfasalazine
Treatment of rheumatoid arthritis with Sulfasalazine should be prescribed by a specialized specialist based on the results of a complete examination of the patient. Most often, at the initial stage, the daily dosage of the drug is 500 mg, which corresponds to one tablet. Every week the dose is increased by 500 mg, bringing the daily requirement to 4-6 tablets. The medicine is taken orally after meals with a sufficient amount of water. Most patients report positive dynamics within 3-6 months from the start of treatment. A lasting therapeutic effect is possible after a year.
Instructions for use of Sulfasalazine for rheumatoid arthritis indicate a number of side effects from certain vital systems. However, the risk of their development does not exceed 10-15%. Among the contraindications, the following can be noted:
- abnormalities in the liver and kidneys;
- obstruction in the genitourinary system or intestines;
- aplastic anemia;
- porphyria;
- allergy to the active ingredient;
- granulocytopenia;
- children under five years of age.
In case of urgent need, the drug is prescribed to pregnant women up to the third trimester.
Many patients ask which drug is more effective for rheumatoid arthritis: Methotrexate or Sulfasalazine. It should be noted that both drugs are classified as basic first-line drugs. Methotrexate is most often involved in the therapeutic strategy. The action of Sulfasalazine is able to control the course of the disease with its moderate and low activity. Sometimes medications are prescribed in combination.
Not everything is so simple: possible problems when using monoclonal antibodies
Monoclonal antibody preparations have been used in rheumatological practice for quite some time. However, they are not prescribed to everyone - not to every first or even every second patient. The main limitation that doctors and patients face is the truly “exorbitant” cost of drugs in this group. Rheumatic diseases cannot be cured in a week or a month - they require many years (or even lifelong) use of therapy. Therefore, when selecting a drug, not only its effectiveness is important, but also its price.
For example, one pack of methotrexate costs approximately 200 rubles. The price of a package of infliximab is about 43 thousand rubles. The difference is obvious. For a year of treatment with methotrexate, even at the maximum dosage, the patient will spend 1–2 thousand rubles on the medicine (depending on the manufacturer, treatment regimen and the cost of the drug in local pharmacies). The price of annual therapy with infliximab is approximately 700 thousand rubles. It is clear that only a very limited group of patients will be able to provide themselves with this medicine.
Therefore, treatment of rheumatic diseases is carried out according to strict algorithms. If a pathology is detected, the patient is prescribed a basic drug. For example, for rheumatoid arthritis, methotrexate will most likely be the main drug. Doctors will add monoclonal antibodies to the standard treatment regimen only in exceptional cases. In Russia, they are considered reserve drugs—additional drugs that should be “left for later,” even despite their high effectiveness. So, if the severity of symptoms does not decrease for a long time (at least 6 months!), a biological drug can be added to methotrexate. Basic therapy is not canceled.
If the disease is initially highly active, progresses rapidly and is accompanied by extra-articular complications, then the patient can immediately be prescribed a combination treatment with basic drugs and monoclonal antibodies. This is due to the fact that biological drugs work best in the “acute period”, when the severity of symptoms is maximum. In addition, the effect of their use is observed faster. Treatment with infliximab produces results within 2–4 weeks, while methotrexate “starts working” only after several months.
The use of biological drugs is permissible in cases where the patient develops intolerance to basic drugs. Patients experience severe side effects from the medication, which further worsens their condition. The use of drugs with a different mechanism of action, including monoclonal antibodies, allows minimizing side effects [18].
The prescription and sale of biological drugs is controlled by the state. Many drugs from the group of monoclonal antibodies (infliximab, etanercept, tocilizumab, golimumab) are included in the “List of Vital and Essential Medicines”. In accordance with it, a list of medications is formed that are supplied to hospitals throughout Russia. Of course, biological drugs are not available in every hospital today. They are usually used in regional centers or specialized hospitals.
If they are unable to provide themselves with medications, patients receive disability and undergo therapy at state expense. This right is enshrined in the current “Program of State Guarantees for the Provision of Free Medical Care.” Treatment with biological drugs is provided for rheumatoid arthritis, ankylosing spondylitis, SLE, dermatopolymyositis, juvenile arthritis and other diseases. At the same time, doctors must determine clear indications for prescribing this or that drug. Getting expensive treatment is quite difficult - you need to undergo a full examination and collect documents. However, the provision of a state quota for many patients is the last chance for a full life.
Another difficulty that can be encountered when using biological drugs is adverse reactions. In parallel with the accumulation of data on the effectiveness of the use of drugs, new undesirable effects from their use are being identified. Most of these reactions are associated with the process of immunosuppression. By suppressing the activity of immune cells, monoclonal antibodies reduce the body's protective function. Anti-infective and anti-tumor immunity are primarily affected [18], [24].
Paradoxically, the use of new drugs against autoimmunity can cause acute autoimmune reactions. All biological drugs are protein molecules that are foreign to the body to one degree or another. Therefore, when therapeutic agents penetrate the patient’s body, the immune system can recognize them as antigens. An active immune response appears - antibodies are produced against the components of the drug.
Autoimmune syndromes provoked by drug administration are usually represented by vasculitis, SLE, antiphospholipid syndrome, and psoriasis [25]. Infliximab, which contains foreign murine fragments, is highly immunogenic. Fully “human” drugs provoke immunity less actively. But even with their use, there is a high risk of developing adverse autoimmune reactions. To eliminate these disorders, it is necessary to adjust the patient’s treatment regimen. It includes additional immunosuppressants that will suppress complications. This may be why combinations of biological drugs with disease-modifying drugs are often more effective than isolated therapy, even with the newest drugs [24].
Despite all possible difficulties, monoclonal antibodies have firmly entered the register of drugs used in rheumatology. The future of biologics and their place in rheumatology will depend on the results of many years of research that remain to be done. But even now we can say that the development of therapeutic monoclonal antibodies is an important step towards defeating autoimmune inflammation.
Sulfasalazine
Side effects are related to the degree of plasma concentration of sulfapyridine, especially in people with slow acetylation. Side effects are more common in patients with rheumatoid arthritis.
From the central and peripheral nervous system: headache, peripheral neuropathy, dizziness, hallucinations, convulsions, ataxia, sleep disturbance, depression, aseptic meningitis.
From the gastrointestinal tract: nausea, vomiting, diarrhea, loss of appetite, pancreatitis, stomatitis, abdominal pain, drug-induced hepatitis.
From the hematopoietic organs: macrocytosis, leukopenia, neutropenia, megaloblastic anemia, hemolytic anemia, hemolytic anemia due to enzyme disorders - with unstable hemoglobin molecules (Heinz-Ehrlich bodies), methemoglobinemia, agranulocytosis, thrombocytopenia, aplastic anemia, hypoprothrombinemia.
From the genitourinary system: proteinuria, hematuria, crystalluria, nephrotic syndrome, transient oligospermia and male infertility.
From the respiratory system: shortness of breath, cough, interstitial pneumonitis, fibrosing alveolitis, infiltrates in the lung tissue.
From the senses: tinnitus.
Laboratory data: hyperbilirubinemia, increased activity of alkaline phosphatase, 'liver' transaminases.
Allergic reactions: generalized skin rash, urticaria, erythema, pruritus, exfoliative dermatitis, photosensitivity, erythema malignant exudative (Stevens-Johnson syndrome), toxic epidermal necrolysis (Lyell's syndrome), fever, lymphadenopathy, serum sickness, periorbital edema, eosinophilia, nodular periarteritis, anaphylactic shock.
Other: hyperthermia, mumps, possible yellow-orange staining of urine, skin or soft contact lenses.
In case of an overdose of the drug, nausea, vomiting, abdominal pain, and dizziness may develop. When using very high doses, anuria, crystalluria, hematuria, and symptoms of toxic damage to the central nervous system (convulsions) may occur. Treatment is symptomatic; it is necessary to induce vomiting, rinse the stomach and intestines; carry out alkalization of urine, forced diuresis. In case of anuria and/or renal failure, fluid and electrolyte intake should be limited.
Literature
- Immunity: fight against strangers and... one's own;
- Systemic lupus erythematosus: a disease with a thousand faces;
- Rheumatoid arthritis: change the composition of the joints;
- Nasonov E.L., Alexandrova E.N., Novikov A.A. (2015). Autoimmune rheumatic diseases - problems of immunopathology and personalized therapy. Bulletin of the Russian Academy of Medical Sciences. 2, 169–182;
- Babaeva A.R., Kalinina E.V., Zvonorenko M.S. (2016). New opportunities to improve the effectiveness and safety of treatment of rheumatoid arthritis. Medical alphabet. 22, 5–12;
- Josef S Smolen, Robert Landewé, Ferdinand C Breedveld, Maya Buch, Gerd Burmester, et. al.. (2014). EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis
.
73 , 492-509; - Nasonov E.L. (2015). Methotrexate for rheumatoid arthritis - 2015: new facts and ideas. Scientific and practical rheumatology. 4, 421–433;
- Kanevskaya M.Z. and Gurskaya S.V. (2013). Methotrexate in the treatment of rheumatic diseases. Modern rheumatology. 4, 47–53;
- D. T. Felson, J. S. Smolen, G. Wells, B. Zhang, L. H. D. van Tuyl, et. al.. (2011). American College of Rheumatology/European League Against Rheumatism Provisional Definition of Remission in Rheumatoid Arthritis for Clinical Trials. Annals of the Rheumatic Diseases
.
70 , 404-413; - Patrick Durez, Jacques Malghem, Adrien Nzeusseu Toukap, Geneviève Depresseux, Bernard R. Lauwerys, et. al.. (2007). Treatment of early rheumatoid arthritis: A randomized magnetic resonance imaging study comparing the effects of methotrexate alone, methotrexate in combination with infliximab, and methotrexate in combination with intravenous pulse methylprednisolone. Arthritis Rheum
.
56 , 3919-3927; - Monoclonal antibodies;
- 12 methods in pictures: immunological technologies;
- Autophagy, protophagy and others;
- Nobel Prize in Medicine or Physiology 2021: for self-criticism;
- Zaichik A.M., Poletaev A.B., Churilov L.P. (2013). Recognition of “One’s own” and interaction with “One’s own” as the main form of activity of the adaptive immune system. Bulletin of St. Petersburg State University. Episode 11. Medicine. 1;
- Autoimmunity. Modern views on the physiological and pathological aspects of autoimmunity. NSU Electronic Archive;
- For the first time in half a century, there is a new drug for lupus;
- Nasonov E.L. and Karateev D.E. (2013). The use of genetically engineered biological drugs for the treatment of rheumatoid arthritis: general characteristics (lecture). Scientific and practical rheumatology. 2, 163–169;
- Logvinenko S.I., Shcherban E.A., Pridachina L.S., Pridachina A.N., Maslova Yu.Yu., Kashichkina A.A. (2016). Genetic engineering in the treatment of ankylosing spondylitis (Bechterew's disease). Scientific bulletins of BelSU. Series: Medicine. Pharmacy. 19, 179–182;
- Masahiko Mihara, Misato Hashizume, Hiroto Yoshida, Miho Suzuki, Masashi Shiina. (2012). IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin.
Sci. .
122 , 143-159; - Nasonov E.L., Alexandrova E.N., Avdeeva A.S., Panasyuk E.Yu. (2013). Inhibition of interleukin 6 - new possibilities for pharmacotherapy of immunoinflammatory rheumatic diseases. Scientific and practical rheumatology. 4, 416–427;
- Suponitskaya E.V., Alexandrova E.N., Aleksankin A.P., Nasonov E.L. (2015). The effect of therapy with genetically engineered biological drugs on B-lymphocyte subpopulations in rheumatic diseases: new data. Scientific and practical rheumatology. 1, 78–83;
- Babaeva A.R., Cherevkova E.V., Galchenko O.E., Solodenkova K.S. (2012). Biological agents in the basic therapy of rheumatoid arthritis. Medicinal Bulletin. 7, 3–9;
- Muravyov Yu.V. and Muravyova L.A. (2016). Untimely thoughts on the use of genetically engineered biological drugs for rheumatic diseases. Scientific and practical rheumatology. 3, 361–366;
- Psoriasis: at war with your own skin.
Pharmacological properties of the drug Sulfasalazine
Like other drugs of the sulfonamide class, Sulfasalazine has certain features:
- good tolerance by the body;
- low likelihood of side effects;
- long period of onset of positive effect;
- reasonable price.
The use of Sulfasalazine in rheumatoid arthritis is due to its anti-inflammatory, antimicrobial and immunosuppressive properties. The active component is able to slow down the development of the disease, even in severe forms of the disease without remission. To prevent rapid progression of the disease, the drug is prescribed in the early stages. The active substance is poorly absorbed by the gastrointestinal tract, due to which it accumulates in the joint fluid. This allows for a local effect directly at the site of inflammation.