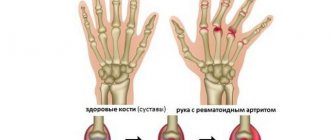
Over the past decade, there has been remarkable progress in the treatment of rheumatoid arthritis , especially when patients develop a form of it that does not respond to traditional antirheumatic drugs.
The greatest achievement was the creation of a group of drugs called biological response modifiers or biologics .
Here are the names of medications approved to safely treat rheumatoid arthritis. They are:
- Actemra;
- Cimzia;
- Enbrel;
- Humira;
- Kineret;
- Orencia;
- Remicade;
- Rituxan;
- Simponi.
On the subject: How to protect your joints if you have rheumatoid arthritis
How do biologics help treat rheumatoid arthritis?
Biologics are genetically engineered proteins derived from human genes. They are designed to suppress specific components of the immune system that play a major role in the formation of inflammation, which is a leading feature of rheumatoid arthritis.
Biologics are used to treat moderate to severe rheumatoid arthritis that is not responding to existing treatments.
Symptoms of rheumatoid arthritis
They differ significantly from traditional drugs for a given disease in that instead of broadly targeting many parts of the immune system, biologics target specific components of the immune system.
Biologic drugs can be taken alone, but are usually prescribed together with other medications for rheumatoid arthritis.
Biologic medications are prescribed to help slow the progression of rheumatoid arthritis after other medications have failed.
- Four effective ways to treat rheumatoid arthritis
Aggressive treatment of the disease is used to prevent the lifelong disability that rheumatoid arthritis causes.
Back to contents
1.What is rheumatoid arthritis and its symptoms?
Arthritis is inflammation in a joint. Joint inflammation causes redness, warmth, swelling and pain in the joint. Rheumatoid arthritis is a type of chronic arthritis that affects both sides of the body (for example, both hands, both wrists, or both knees). This symmetry helps differentiate rheumatoid arthritis from other types of arthritis.
Rheumatoid arthritis can harm more than just your joints. It can affect the skin, eyes, lungs, heart, blood and nervous system.
Side effects of biologics?
The most common side effect of biologics is pain and rash at the injection site, although these symptoms occur in less than 30% of patients.
If biological products are administered intravenously, there is a possibility of an allergic reaction, so the patient’s condition is monitored during the infusion.
Symptoms of an allergic reaction to IV fluids include:
- fever,
- chills,
- nausea,
- headache.
Just like other medications that suppress the immune system, biologics can increase your risk of contracting an infection or other illnesses.
Patients taking biologics should contact their doctor immediately if they have a persistent fever or unexplained symptoms.
Vaccinations that prevent infections should be done before taking biological products, since vaccination is excluded during the course of biological therapy.
Biological drugs can also cause chronic diseases (for example, tuberculosis ), which in the early stages proceed unnoticed.
Tuberculosis: topic overview
- Effective new generation drugs in the treatment of rheumatoid arthritis
They are not recommended for people with multiple sclerosis and certain other conditions, such as severe heart failure. All patients need to have a skin test for tuberculosis before starting biotherapy, and many also need to be tested for chronic hepatitis.
The price of biological products is higher than that of conventional drugs. However, there is evidence to suggest that they are more effective and carry fewer risks than other systemic treatments.
A significant disadvantage of biologics is that they must be introduced into the body by injection or intravenous infusion, with the exception of Xeljanz, which is taken orally.
Although animal studies have not shown any effect of biologics on fertility or fetal impairment, these results may not necessarily predict the effect they will have in humans.
Pregnant women should take these medications only when absolutely necessary, as the possible effects of these medications on a developing baby have not been well studied.
Biotherapy is temporarily stopped before surgery until the wounds are healed and the risk of infection has passed. As a general rule, different biologics should not be taken at the same time.
On the subject: How to get rid of pain from rheumatoid arthritis? Back to contents
Review of biologics for rheumatoid arthritis
Here is some information about the biologics available today:
Actemra
Actemra is administered monthly over hour-long infusions or weekly (or biweekly) injections. The drug is the first IL-6 inhibitor for rheumatoid arthritis. IL-6 or Interleukin-6 is a chemical messenger of the immune system.
Actemra is used to treat moderate to severe rheumatoid arthritis that is resistant to one or more TNF inhibitors, which include Cimzia, Enbrel, Humira, Remicade and Simponi.
- Some issues in the treatment of rheumatoid arthritis with concomitant chronic hepatitis B or C
The most common side effects of this drug are:
- upper respiratory tract infections,
- inflammation of the nose or throat,
- headache,
- high blood pressure,
- elevated levels of liver enzymes.
Cimzia
Cimzia works by blocking the action of a body substance called tumor necrosis factor (TNF). The drug is introduced into the body through injections.
Instruction from a healthcare professional will allow the patient to administer the injections independently. After the initial doses, this biological product can be introduced into the body every 2-4 weeks, depending on the dosage prescribed by the attending physician.
In addition to pain at the injection site, the most common side effects of Cimzia include:
- upper respiratory infections (for example, influenza),
- headache,
- high blood pressure,
- inflammation of the nose or throat,
- back pain.
Enbrel
Enbrel reduces joint inflammation and the damage caused by rheumatoid arthritis by blocking an inflammatory chemical called tumor necrosis factor (TNF).
Enbrel is also an injectable drug. It is injected into the body subcutaneously twice or once a week. Patients can learn to administer the injections themselves or have family members or caregivers who have received the necessary instructions.
The drug is available in the form of a solution in syringes for one-time use, which makes it convenient for self-injection.
Besides pain at the injection site, the most common side effects are:
- infections (such as flu),
- diarrhea,
- rash and itchy skin.
Humira
Humira reduces joint inflammation and the damage caused by rheumatoid arthritis by blocking an inflammatory chemical called tumor necrosis factor (TNF).
Humira is an injectable biological product. It is injected into the body subcutaneously twice or once a week. Patients can learn to administer the injections themselves or have family members or caregivers who have received the necessary instructions.
The drug is available in the form of a solution in syringes for one-time use, which makes it convenient for self-injection.
Common side effects of Humira, other than pain at the injection site, include:
- upper respiratory infections (including sinusitis),
- headache,
- rash,
- nausea,
- back pain.
Kineret
Kineret is a protein that reduces joint inflammation by blocking the action of the chemical messenger interleukin-1. This biological product is administered one injection per day (which can be done independently or with outside help).
In addition to pain at the injection site, side effects of Kineret include upper respiratory infections (including sinusitis), headache, nausea and diarrhea.
Orencia
Orencia is a protein that blocks signals needed to activate the immune system's T cells. Activated T cells play an important role in the development of rheumatoid arthritis.
Orencia can be given by intravenous infusion monthly or by injection every week.
The main side effects of this drug are:
- headache,
- inflammation of the nose or throat,
- dizziness,
- cough,
- backache.
Remicade
Like Enbrel and Humira, Remicade reduces inflammation and the damage caused by rheumatoid arthritis by blocking the chemical activator of inflammation, tumor necrosis factor (TNF).
Remicade is given to the body through an intravenous infusion at a doctor's office, infusion center, or hospital. Each infusion takes approximately 2 hours. This procedure is repeated 3 times during the first six weeks of therapy, then every eight weeks.
The most common side effects of Remicade are:
- upper respiratory tract infections (including sinusitis),
- nausea,
- headache,
- abdominal pain,
- diarrhea.
Rituxan
Rituxan is used to treat rheumatoid arthritis that has not responded to TNF blocking drugs such as Enbrel, Humira, or Remicade.
This biologic is an antibody—a protein that monitors and reduces the number of specialized white blood cells called B cells.
Rituxan is administered into the body in two intravenous infusions two weeks apart. A repeat course can be taken after four or six months.
The most common side effects of Rituxan are:
- fever,
- chills,
- nausea,
- headache,
- low white blood cell count (leukopenia).
Simponi
This biologic blocks TNF, which causes inflammation. Simponi is administered once a month by injection or intravenously.
The drug is available in the form of a solution in syringes for one-time use, which makes it convenient for self-injection.
Besides a reaction at the site of infection, side effects of Simponi include:
- upper respiratory tract infections,
- runny nose,
- abnormal liver test results,
- high blood pressure.
Back to contents
Glucocorticoids for rheumatoid arthritis: pros and cons
A little glucocorticoid, like a glass of wine, can be beneficial to many (patients), a lot of glucocorticoid, like a bottle of wine, is harmful to everyone.
T. Pincus
M
The role of glucocorticoids (GCs) in the treatment of rheumatoid arthritis (RA) has been widely discussed in the rheumatological community for more than 50 years [1–7].
The discovery of the extremely high effectiveness of GCs in RA was a breakthrough in the treatment of this disease and served as the basis for awarding the Nobel Prize in Medicine to American scientists Hench, Kendal and Reichsten in 1950 [8]. However, it very soon became obvious that the inevitably developing severe toxic reactions with long-term use of high (more than 20 mg) doses of GCs in RA completely neutralize the beneficial effect of treatment [9]. Moreover, according to an analysis of the database (Arthritis, Rheumatism, and Aging Medical Information System - ARAMIS
), in RA patients treated with GCs, the risk of premature mortality is 1.5 times higher than in patients who did not receive GCs [10].
Already since the late 50s, it has become an axiom for rheumatologists that the prescription of high doses of GC is justified only for very strict indications:
- potentially fatal complications of rheumatoid vasculitis
- very high activity of the rheumatoid process
- severe complications of therapy with basic anti-inflammatory drugs.
In other cases, prescribing high doses of GCs for RA and for a long time is absolutely contraindicated and can be considered a serious medical error.
However, there has been a heated debate about the place of “low” doses of GC in the treatment of “early” RA [1–3,7]. It should be recalled that, according to modern nomenclature, “low” means a dose of GC < 7.5 mg/day (in terms of prednisolone), “medium” > 7.5 mg/day – 30 mg/day, and “high doses” – > 30 mg/day [11], and under “early” RA – the duration of the disease is no more than 12 months.
According to modern concepts, RA is a classic model of chronic human inflammatory disease, the pathogenesis of which is based on severe disorders in the immune system [12]. Conventionally, two closely interrelated, but partially occurring independently of each other pathological processes are distinguished - synovitis (infiltration of synovial tissue by activated lymphocytes) and the formation of bone erosions (infiltration of synovial tissue by activated macrophages). Being purely conditional, this division has a certain pathomorphological justification and important clinical significance. If synovial inflammation can, in principle, be controlled by non-steroidal anti-inflammatory drugs (NSAIDs), then the destructive process can only be controlled by basic anti-inflammatory drugs, and probably also by GC.
Rationale for the use of GCs in early RA
GCs are still the most effective anti-inflammatory drugs and have the potential to suppress most of the mechanisms underlying rheumatoid inflammation [3] (Table 1).
It is also believed that in patients with “early” RA and even in relatively healthy people with the “preclinical” stage of RA, there is a “hidden” insufficiency of the “hypothalamus-pituitary-adrenal” axis, which manifests itself in the inadequate synthesis of endogenous cortisol in response to “stressors” incentives. This defect may contribute to the chronicity of rheumatoid inflammation [13,14]. Therefore, treatment with low doses of GC can be considered as a unique form of “replacement” therapy aimed at correcting adrenal insufficiency.
According to modern concepts, early aggressive therapy can significantly improve the long-term prognosis of RA
[15]. Moreover, only GCs and, probably, tumor necrosis factor (TNF)-a inhibitors are able to quickly suppress rheumatoid inflammation, while the effect of “basic” anti-inflammatory drugs requires a longer time (from several weeks to months). If indeed active anti-inflammatory therapy in the early stage of RA (“therapeutic window”) is so important for improving long-term prognosis, this may be an additional argument for prescribing GCs at the onset of the disease.
Thus, the use of GCs in RA is theoretically very well justified, but the practical results of GC treatment are not so encouraging. To date, very few controlled studies have been conducted to evaluate the effectiveness of GCs in early RA, meeting the criteria of “evidence-based medicine” [1–4,16]. The frequency and severity of side effects of GCs and their negative “contribution” to disease outcomes have also been insufficiently studied [1,7]. In fact, significantly fewer studies have been devoted to assessing the true significance of the negative effects of GC therapy depending on treatment regimens and clinical features of RA than to studying their effectiveness.
Let us consider just some of the arguments for and against the use of GCs in RA, as well as modern recommendations regarding the prevention of the main complications of glucocorticoid therapy.
Clinical effectiveness
In 1997, a Cochrane review based on an analysis of 7 studies, including 253 patients, reported [17]. The results of more recent studies also indicate that the administration of GC in low doses (< 10 mg/day) in combination with disease-modifying anti-inflammatory drugs can achieve rapid clinical effect (compared with placebo). However, the effect of GC monotherapy is quite short-lived and lasts no more than 6 months [18–20]. In the recommendations of the American College of Rheumatology (2002) [21], Fr.
Radiographic progression
According to modern concepts, an important “end point” that allows assessing the long-term prognosis of the disease is the radiological progression of joint destruction. Slowing progression is included in the official indications for the new “background” anti-inflammatory drugs such as leflunomide, etanercept and infliximab. Therefore, the question of whether GC therapy affects joint destruction is of particular interest.
Since 1980, 10 controlled studies (involving more than 700 patients) have been conducted to assess the “baseline” effect of GCs. The results of most studies indicate that GCs have the ability to slow down the radiographic progression of joint destruction, and this effect persists for a long time after discontinuation of GCs (Table 2).
Of particular interest are the results of the
COBRA
[24], in which the effectiveness of combination therapy with methotrexate (MTX), sulfasalazine and prednisolone (initial dose of 60 mg/day followed by a decrease to 7.5 mg/day over a period of time) was compared in patients with “early” RA. 7 weeks) compared with sulfasalazine monotherapy. In the process of prospective observation, it was convincingly shown that although after discontinuation of prednisolone the compared groups did not differ in clinical criteria of activity, patients receiving prednisolone had a less pronounced progression of the erosive process in the joints, and this effect persisted for at least 5 years prospective observation [26]. Similar data on the “basic” effect of GC monotherapy in “early” RA were demonstrated in the only double-blind, placebo-controlled study [19].
Our results indicate that low doses of GC in combination with MT significantly reduce clinical and laboratory manifestations of activity in early RA, and the effectiveness of therapy according to ACR criteria by 12 months. treatment was significantly higher than in patients receiving MTX monotherapy [27] (Fig. 1). During combination therapy with GC and MT, a significant slowdown in the radiological progression of the erosive process in the hands and feet was observed compared with MT monotherapy (p<0.05).
Rice. 1. Efficacy of MTX and combination therapy of MTX and GC in RA
Thus, it has been proven that in “early” RA, the use of GCs can achieve the following main positive effects [4]:
- reduce joint pain, improve functional activity
- slow down the radiographic progression of joint destruction that persists after cessation of GC use
- reduce the need for NSAIDs
- increase the effectiveness of basic anti-inflammatory therapy.
Side effects
Side effects of GCs can be divided into two groups – potentially controllable and uncontrollable (Table 3), as well as “fast”, “slow” and “idiosyncratic”.
When assessing the “clinical significance” of side effects of GCs, it should be emphasized that although most of them are quite severe and are an inevitable consequence of glucocorticoid therapy, some develop less frequently than during treatment with other (symptomatic and basic) anti-inflammatory drugs. It is necessary to take into account that uncontrolled rheumatoid inflammation itself can lead to the development of a number of severe complications, which to a certain extent resemble the side effects of GC. This suggests that some complications of RA, which are considered to be a consequence of GC treatment, are actually associated with inadequate glucocorticoid therapy. In our opinion, inadequate glucocorticoid therapy means the use of too high or, on the contrary, too low doses, unjustified duration of treatment, glucocorticosteroid monotherapy without the combined use of effective basic anti-inflammatory drugs with “steroid-sparing” activity. Let's look at just a few of the most typical, from our point of view, examples.
GCs are less likely to cause severe gastrointestinal damage than nonsteroidal anti-inflammatory drugs (NSAIDs). The twofold increase in the risk of developing gastric ulcers described during GC treatment is believed to be associated with concomitant use of NSAIDs [28].
Data regarding the role of GC in the development of atherosclerotic vascular lesions are contradictory. Since the inflammatory activity of RA is an important “pro-atherogenic” factor [29], suppression of inflammation, including with the help of GCs, can potentially reduce the risk of developing atherosclerosis. Recent studies have shown that GCs can help normalize lipid abnormalities associated with high inflammatory activity of RA [30].
Although treatment with GCs may lead to hyperglycemia requiring correction, the risk of its development decreases as the duration of GC use increases (from 1.7 during the first 45 days to 1.1 after 90 days) [31]. Moreover, there is evidence that in RA the development of insulin resistance correlates with inflammatory activity [32], and anti-inflammatory therapy, including glucocorticoid therapy, leads to the restoration of insulin sensitivity [33].
Treatment with GCs is associated with an increased risk of infectious complications, but this is observed primarily in patients receiving high doses of GCs. For example, according to AE Stuck et al. [34], in patients with rheumatic diseases receiving prednisolone at an average dose of 8 mg/day, there is no increase in the incidence of infectious complications.
There is no doubt that the use of GCs, even in low doses (<8 mg/day), leads to transient suppression of the hypothalamic-pituitary-adrenal axis. However, for how long GCs must be taken (and in what doses) to cause persistent adrenal insufficiency is not yet known. Moreover, as already noted, the development of an immunopathological process in RA is associated with suppression of the synthesis of endogenous cortisol [35].
Treatment with GCs in RA, as a rule, is not accompanied by severe mental disorders (steroid psychosis) [36], although it can lead to dysfunction at the level of the hippocampus, manifested in memory impairment [37].
One of the proven side effects of GC therapy is the development of cataracts [38], however, the effect of the dose and duration of GC therapy on the risk of developing this complication in RA has not yet been specifically analyzed.
Glucocorticoid osteoporosis
According to modern concepts, the most unfavorable consequence of long-term GC therapy is osteoporosis
[39]. The risk of osteoporetic fractures in RA patients receiving relatively small doses of GCs (average 8.6 mg/day) reaches 33% over 5 years [40]. Another study showed that the relative risk of osteoporosis in RA patients treated with GCs was 2.6 for the femur and 2.7 for the spine [41]. At the same time, according to a number of authors, the inflammatory activity of RA and impaired physical activity are no less important risk factors for osteoporosis than treatment with GCs [42,43]. Therefore, in some patients with RA, GCs may actually have an indirect beneficial (antiosteoporotic) effect on bone tissue, which is associated with suppression of the inflammatory component of the pathogenesis of osteoporosis [43]. According to our data, although in patients receiving GC in combination with MT after 12 months. BMD values in the spine and femoral neck were lower than in patients receiving only MT; these differences (as well as the relative severity of negative BMD dynamics) were not statistically significant (p>0.05) (Fig. 2).
Rice. 2. Dynamics of BMD in the lumbar spine (A) and in the femoral neck (B) during treatment with MT and combined therapy with MT and GC
Nevertheless, the fact that osteoporetic fractures are the most severe consequences of inadequate glucocorticoid therapy is beyond doubt [44]. Another potential severe complication of glucocorticoid therapy is osteonecrosis, which develops much less frequently than osteoporosis (less than 3% of patients taking low-dose glucocorticosteroids) [45].
According to international recommendations, BMD determination using bone densitometry should be performed in all patients who are planned to be treated with GC, regardless of dose, for more than 6 months (category of recommendations for clinical practice A) [46]. In patients not receiving antiosteoporetic therapy, densitometry should be repeated every 6 months, and in those receiving this therapy - at least once a year.
In order to reduce the risk of developing osteoporosis, lifestyle changes are important, namely quitting smoking and drinking alcohol, regular exercise, eating foods high in calcium and vitamin D, and regular sun exposure. Recommendations regarding pharmacotherapy for osteoporosis are summarized in Table 4 and Figure 3.
Rice. 3. Algorithm for pharmacotherapy of glucocorticoid osteoporosis
An important place in the prevention and treatment of glucocorticoid osteoporosis is occupied by the use of
calcium and vitamin D preparations
, the clinical and pharmacoeconomic effectiveness of which has been confirmed in numerous studies [46–48]. In fact, calcium and vitamin D supplementation are recommended for all RA patients taking GCs [49]. Active metabolites of vitamin D (alfacalcidol) are undoubtedly effective, however, during treatment with this drug, careful monitoring of hypercalcemia and hypercalciuria is necessary [50,51].
One of the most effective methods of treating GC-osteoporosis is pharmacotherapy with salmon calcitonin preparations (Miacalcic)
. Along with antiosteoporetic, calcitonin has pronounced analgesic activity, which makes it especially attractive in patients with spinal pain associated with osteoporetic fractures [52]. After just a single use of Miacalcic nasal aerosol, a person experiences a clinically significant biological response, which is manifested by an increase in urinary excretion of calcium, phosphorus and sodium (due to a decrease in their tubular reabsorption) and a decrease in hydroxyproline excretion. Long-term use of Miacalcic leads to a significant and long-term (within 5 years of treatment) decrease in the level of biochemical markers of bone metabolism, such as serum C-telopeptides (sCTX) and bone isoenzymes of alkaline phosphatase. The use of Miacalcic nasal aerosol leads to a statistically significant increase (by 1–2%) in bone mineral density in the lumbar vertebrae, which is determined already in the first year of treatment and lasts up to 5 years. Miacalcic ensures the maintenance of mineral density in the femur. The use of Miacalcic nasal aerosol at a dose of 200 IU per day leads to a statistically and clinically significant reduction (by 36%) in the risk of developing new vertebral fractures in the group of patients receiving Miacalcic (in combination with vitamin D and calcium preparations), compared with the group of patients receiving Miacalcic (in combination with vitamin D and calcium preparations). receiving placebo (in combination with the same drugs). In addition, in the group of patients treated with Miacalcic (in combination with vitamin D and calcium preparations), compared with the group of patients receiving placebo (in combination with the same drugs), a 35% decrease in the incidence of multiple vertebral fractures was noted.
Bisphosphonates are effective drugs for glucocorticoid osteoporosis
, which are potent inhibitors of bone resorption. During treatment with these drugs, there is not only a significant increase in BMD, but also a significant decrease in the risk of osteoporetic fractures of the spine. The disadvantages of bisphosphonates include the fact that their use is relatively contraindicated in women before menopause. In addition, there is evidence that the combined use of bisphosphonates and NSAIDs can potentially significantly increase the risk of ulcerative necrotic lesions of the gastrointestinal tract (esophagus, stomach).
The effectiveness of other drugs (fluorides, anabolic drugs, parathyroid hormone peptides, etc.) has not been strictly proven. It is believed that “aggressive” prevention and treatment of glucocorticoid osteoporosis will significantly improve the results of treatment of RA with GCs and will make the use of GCs in RA more justified from the point of view of the prognosis of the disease.
Thus, GCs still remain an important method of pharmacotherapy for RA.
However, GC treatment can only be initiated after a comprehensive assessment of the pros and cons of glucocorticoid therapy and a serious discussion with the patient about its possible consequences. Prescribing GC to patients with RA is a responsible decision that can only be taken by a qualified rheumatologist. References:
1. Conn DL. Resolved: low–dose prednisone is indicated as a standard treatment in patients with rheumatoid arthritis. Arthritis Care&Res 2001; 45:462–467
2. Saag KG. Resolved: low-dose glucocorticoids are neither safe nor effective for long-term treatment of rheumatoid arthritis. Arthritis Care&Res 2001; 45:468–471
3. Moreland LW, O`Dell JR. Glucocorticoids and rheumatoid arthritis. Back to the future? Arthritis Rheum 2002; 40:2553–2563.
4. Conn DL, Lim SS. New role for an old friend; prednisone is a disease–midifying agent in early rheumatoid arthritis. Curr Opin Rheumatol 2003; 15:193–198
5. Nasonov E.L. Treatment of rheumatoid arthritis: the role of glucocorticoids. Klin Med 1999;4: 4–8
6. Pincus T, Sakka T, Stein CM. Are long–term very low doses of prednisolone for patients with rheumatoid arthritis as helpful as high doses are harmful? Ann Intern Med 2002; 136; 76–78.
7. Bijsma JW, Boers M, Saag KG, Furst DE. Glucocorticoids in the treatment of early and late RA. Ann Rheun Dis 2003; 62:1033–1037.
8. Hench PS, Kensall EC, Slocumb CH, Polley HF. The effect of a hormone of the adrenal cortex (17–hydroxy–11–dehydrocortisone; compound E) and the pituitary adrenocorticotropic hormone on rheumatoid arthritis. Proc Staff Meet Mayo Clin 1949; 24:181
9. Bollet AJ, Black R, Bunim JJ. Major undesirable effects resulting from prednisolone and prednisone. JAMA 1955; 158:459–463.
10. Wolfe F, Mitchell DM, Sibley JT, et al. Themortality of rheumatoid arthritis. Arthritis Rheum 1994; 37:2529–2533.
11. Buttgereit F, Da Silva JAP, Boers M, et al. Standardized nomenclature for glucocorticoid dosage and glucocorticoid treatment regimes: current questions and tentative answers in rheumatology. Ann Rheum Dis 2002; 61:718–722.
12. Firenstein GS. Evolving concepts of rheumatoid arthritis. Nature 2003; 423:356–361
13. Dekker JC, Greenen R, Godaert GLR. Bjisma JWL. Diulnar rhythm of salivary cortisol in patients with RA. Arthritis Rheum 2000; 43:465–467
14. Cutolo M, Foppiani L, Prete C, et al. Hypothalamic–pituitary–adrenocortical axis in premenopausal rheumatoid arthritis not treated with flucocorticoids. J Rheumatol 1999; 26:282–288.
15. Nasonov E.L. Why is early diagnosis and treatment of rheumatoid arthritis necessary? RMJ 2002; 10 (22);1009–1014
16. Suponitskaya EV. The effect of low doses of glucocorticoids on joint destruction and bone mineral density in early rheumatoid arthritis. Scientific and Practical Rheumatology 2003; 4: 78–81.
17. Criswell LA, Saag KG, Sems KM, Welch V, et al. Moderate–term, low–dose corticosteroids for rheumatoid arthritis. Cochrane Database Syst Rev 2000; CD01158.
18. Hansen M, Podenphant J, Florescu A, et al. A randomized trial of differentiated prednisolone treatment in active rheumatoid arthritis. Clinical benefits and skeletal side effects. Ann Rheum Dis. 1999; 58:713–718
19. Van Everdinger AA, Jacobs JWG, et al. Low dose prednisolone therapy for patients with early active rheumatoid arthritis; clinical efficacy, disease modifying properties and side effects: a randomizes double0blind placebo–controlled trial. Ann Intern Med 2002; 136:1–12
20. Kirwan JR. Arthritis and Rheumatism Council Low-dose glucocorticoid study group. The effect of glucocorticoids on joint destruction in rheumatoid arthritis. N Engl J Med 1995; 333; 142–145.
21. ACR Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for management of RA; 2002 update. Arthritis Rheum 2002; 46: 328–246.
22. Harris ED Jr, Emkey RD, Nichols JE, Newbwerg A. Low dose prednisolone therapy in rheumatoid arthritis: a double blind study. J Rheumatol 1983; 19: 1520–1526.
23. Milliom R, Poole P, Kellegren JH, Jayson MI. Long–term study of management of rheumatoid arthritis. Lancet 1984; 1:812–816
24. Boers M, Verhoeven AC, Markusse HM, et al. Randomized comparison of combined step–down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet 1997; 350:309–318.
25. Paulus HE, di Primeo D, Sanda M, et al. Progression of radiographic joint erosion during low dose corticosteroid treatment of rheumatoid arthritis J Rheumatol 2000; 27:1632–1637.
26. Landewe RB, Boers M, Verhoeven AC, et al. COBRA combination therapy in patients with early rheumatoid arthritis: long–term structural benefits of a brief intervention. Arthritis Rheum 2002; 46: 347–356.
27. Suponitskay EV, Nassonov EL, Smirnov AV, Demin NV. Effects of low dose glucocorticoids in the treatment of early active rheumatoid arthritis. Results of the 1st year srudy. Ann Rheum Dis 2003; 62 (Suppl 1): THUO190:167 (abst).
28. Piper JM, Ray WA, Daugherty JR, Griffin MR. Corticosteroid use and peptic ulcer: role of non-steroidal anti-inflammatory drugs. Ann Intern Med 1991; 114:735–740.
29. Sattar N, McCarey DW, Capell H, McInnes IA. Explaining how “high-grade” systemic inflammation accelerated vascular risk in rheumatoid arthritis. Circulation 2003; 108:2957–2963.
30. Boers M, Nurmahamed MT, Doelman CJA, et al. Influence of glucocorticoids and disease activity on total and high density lipoprotein cholesterol in patients with rheumatoid arthritis. Ann Rheum Dis 2003; 62:842–845.
31. Gurwitz JH, Bohn RL, Glynn RJ, et al. Glucocorticoids and the risk for initiation of hypoglycemic therapy. Arch Intern Med 1994; 154:97–101.
32. Dessein PH, Stanwix AE, Joffe BI. Cardiovascular risk in rheumatoid arthritis versus osteoarthritis: acute phase response related decrease in insulin sensitivity and high–density lipoprotein cholesterol as well as clustering of metabolic syndrome features in rheumatoid arthritis. Arthritis Res 2002; 4:R5–R12.
33. Halgren R, Berne C. Glucose intolerance in patients with chronic inflammatory diseases is normalized by glucocorticoids. Acta Med Scand 1983; 213:351–355.
34. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis 1989; 11:954–963.
35. Bijsma JWJ, Straub RH, Masi AT, et al. Neuroendocrine immune mechanisms in rheumatic disease. Trends Immunol 2002; 23:59–61
36. Wolkowitz OM, Reus VI, Canick J, et al. Glucocorticoid medication. Memory and steroid psychosis in medical illness. Ann NY Acad Sci 1997; 823:81–96.
37. Keenan PA, Jacobson MW, Soleymani RM, et al. Commonly used therapeutic doses of glucocorticoids impair explicit memory. Ann NY Acad Sci 1995; 761:400–402
38. Carnahan MC, Goldstein DA. Ocular complications of topical, peri–ocular, and systemic cortocosteroids. Curr Opin Ophthalmol 2000; 11:478–483
39. Nasonov E.L. Glucocorticoid osteoporosis: modern recommendations. Consilium, 2002; 4(8): 403–408
40. Michel BA, Bloch DA, Fries JF. Predictors of fracture in early rheumatoid arthritis. J Rheumatol 1991; 18:804–808.
41. Haugeberg G, Orstavic RE, Uhlig T, et al. Bone loss in patients with rheumatoid arthritis: results from a population–based cohort of 366 patients followed up for two years. Arthritis Rheum 2002; 46:1720–1728.
42. Jolles BM, Boloch ER. Current consensus recommendations for rheumatoid arthritis therapy: a blind spot for osteoporosis prevention and treatment J Rheumatol 2002
43. Verhoeven A, Boers M. Limited bone loss due to cortocosteroids: a systematic review of prospective studies in rheumatoid arthritis and other disease. J Rheumatol 1997; 24:1495–1503.
44. Van Staa T, Leufkens H, Abenhaim L, et al. Use of oral corticosteroids and risk of fracture. J Bone Miner Res 2000; 15:993–1000.
45. Vreden SG, Hermus AR, van Liessum PA, t al. Aseptic bone necrosis in patients on glucocorticoid replacement therapy. Neth J Med 1991; 114:735–740
46. American College of Rheumatology ad Hoc committie on glucocorticoid–induced osteoporosis. Recommendation for the prevention of glucocorticoid–induced osteoporosis. Arthritis Rheum 2001; 44:1496–1503
47. Brown JP, Josse RG, for the Scientific Advisory Council of the Osteoporosis Society of Canada. Canadian guidelines for osteoporosis. CMAJ 2002; 167 (Suppl 10): S1–S34
48. Yeap SS, Hosking DJ. Management of corticoid–induced osteoporosis. Rheumatology 2002; 41:1088–1094.
49. Amin S, LaValley MP, Simms RW, Felson DT. The role of vitamin D in corticosteroid–induced osteoporosis: a meta–analytic approach. Arthritis Rheum 1999; 42:1740–51
50. Jehle PM. Steroid-induced osteoporosis: how can it be avoided? Nephrol Dial Transplant 2003; 18:861–864.
51. Nasonov EL, Ghukasyan YES. Use of alfacalcidol (alfv-D3-Teva) for the prevention and treatment of osteoporosis. Therapist. archive 2000, 11, 71–73.
52. Cranney A, Welch V, Adachi JD, Homik J, Shea B, Suarez–Almazor ME, et al. Calcitonin for the treatment and prevention of corticosteroid–induced osteoporosis Cochrane review. In: The Cochrane Library. Issue 2. Oxford (UK): Update Software; 2000
What are biological products
Biological drugs can be introduced into the body through self-injection
About twenty years ago, the US Food and Drug Administration approved the first biologic to treat rheumatoid arthritis. Since then, the organization has allowed doctors to use 8 more similar drugs, as well as new synthetic drugs.
In simple terms, biological drugs or biological agents are proteins that have been genetically modified to neutralize those immune system cells that attack healthy tissue and thereby cause the symptoms of rheumatoid arthritis.
Biological agents belong to the range of targeted or targeted therapies. This is how they differ from other drugs for RA, which in medical practice are usually called traditional. An example of such drugs is methotrexate, which does not attack specific cells.
The authors of an article published in 2021 by the scientific journal Clinical Pharmacology & Therapeutics indicated that biologics can slow the progression of joint damage caused by rheumatoid arthritis.
Biologics are not always offered as first-line treatment for RA. Many patients with this condition experience symptoms due to problems with multiple types of immune cells, and biological agents may only target one type.
Although doctors can prescribe several biological drugs at once, with each new drug the number of side effects that occur increases.
Sometimes the doctor prescribes treatment with biological drugs along with traditional medications. In these situations, finding the most effective combination to combat your unique symptoms may take some time.
Who can get rheumatoid arthritis?
According to statistics, rheumatoid arthritis occurs in approximately 1% of people. It is an autoimmune disease in which the body's immune system attacks its own healthier cells and tissues. Rheumatoid arthritis is two to three times more common in women than men, but symptoms in men tend to be much more severe. Rheumatoid arthritis usually develops in middle age, but the disease also occurs in young children and older adults.
A must read! Help with treatment and hospitalization!
Types of biological products
One example of a biologic drug is rituximab.
Doctors classify biological agents by the type of cells they interact with. Listed below are the names of pharmaceutical groups with a brief description.
B-lymphocyte inhibitors
- Example. Rituximab (Rituxan).
- Operating principle. Drugs in this class destroy B-lymphocytes that cause inflammation. They must be given intravenously in two sessions every 6 to 12 months. One session lasts from 4 to 6 hours. Patients who are treated with B-lymphocyte inhibitors through intravenous infusions are at risk of developing infections and.
Interleukin-1 blockers
- Example. Anakinra (Kinneret).
- Operating principle. Interleukin-1 blockers, or IL-1 blockers, interact with interleukin, an inflammatory compound found in the body. These drugs are prescribed less often than other biological agents intended to treat rheumatoid arthritis. Patients self-administer IL-1 blockers on a daily basis.
Interleukin-6 inhibitors
- Examples. Sarilumab (Kevzara), tocilizumab (Actemra).
- Operating principle. These drugs prevent interleukin-6 proteins from interacting with cells and thus prevent inflammation. Sarilumab is an injection that people must receive every two weeks. Tocilizumab is another drug in this group, administered intravenously once a month.
T-lymphocyte inhibitors
- Example. Abatecept (Orencia).
- Operating principle. Such drugs attach to the surface of T-lymphocytes, that is, white blood cells that cause inflammation. Doctors give T-cell inhibitors intravenously over 30 minutes every other week for a month and a half. After this, patients begin receiving infusions every four weeks.
Tumor necrosis factor inhibitors (TNF inhibitors)
- Examples. Adalimumab (Humira), certolizumab (Cimzia), golimumab (Simponi); infliximab (Remicade).
- Operating principle. These drugs are able to suppress the activity of tumor necrosis factor (TNF), which is responsible for the onset of the stages of inflammation. TNF inhibitors are prescribed by doctors more often than other drugs because they have been used for quite a long time to treat rheumatoid arthritis. Infliximab is administered intravenously, while other drugs can be used by self-injection.
Another biologic is tofacitinib (Yaquinus). It interacts with proteins that cause rheumatoid arthritis called Janus kinases. However, this drug targets proteins that are inside cells, while all other drugs act on proteins located outside of cells.
Unlike other biologics, which must be given by intravenous injection, tofacitinib can be taken in tablet form twice a day.
3.Diagnosis and treatment of the disease
Diagnosis of rheumatoid arthritis
Several methods are used to diagnose rheumatoid arthritis :
- Analysis of arthritis symptoms - symmetry of painful joints, joint stiffness in the morning, pain, etc. Formation of rheumatoid nodules under the skin;
- X-ray examination;
- Blood tests.
Most people with rheumatoid arthritis (but not all) have a specific antibody (rheumatoid factor) in their blood. This rheumatoid factor can also be present in the blood of people who do not have arthritis. Therefore, the diagnosis of rheumatoid arthritis cannot be made based on this test alone. Other antibodies found in the blood may indicate an aggressive form of rheumatoid arthritis or, for example, an autoimmune disease in general.
With arthritis, moderate anemia can develop, which means that this will also be visible in a blood test, just like inflammation.
Treatment of rheumatoid arthritis
There are many ways to treat rheumatoid arthritis. It may include a combination of medications, rest and exercise, and surgery to correct joint damage. The specific treatment regimen for arthritis depends on the patient's age, general health, medical history, and severity of the arthritis.
Many medications can help with joint pain, swelling and inflammation. Some drugs can prevent or slow the progression of the disease. Your doctor may recommend anti-inflammatory pain medications such as aspirin or ibuprofen, as well as pain-relieving ointments (creams), corticosteroids, or narcotic pain relievers. Potent antirheumatic drugs that suppress the immune system and its attack on the joints are also used to treat rheumatoid arthritis.
If drug therapy does not provide the desired results, joint surgery
.
How are biological drugs used?
With the exception of tofacitinib, most biologic drugs are administered to the body through intravenous infusions or self-injections.
These products are not available in tablet form because they contain molecules that are considered too small to reach the bloodstream. Therefore, doctors inject them directly into the blood or into areas of the body that are close to the bloodstream. The doctor can discuss with the patient the method of administering the medication, as well as the schedule for taking infusions or giving injections.
Some biological products have an effect on the body almost immediately after entering the body, while others show their effect after a long time.
Side effects of biologics
The most common side effect of injected or infused biological agents is the development of infection. The introduction of such agents can irritate the skin and cause the penetration of bacteria and viruses into the body, which are potential causative agents of infectious diseases.
Very rare side effects of biologics include swelling of the ankles and hands.
In addition, biological agents affect cells of the immune system, as a result of which the body's ability to resist infections may be impaired.
Very rare side effects of biologics include the following:
- demyelination syndrome, which is characterized by damage to nerve fibers and sometimes causes visual disturbances;
- development of symptoms characteristic of lupus;
- reactivation of hepatitis B in people who have had hepatitis B;
- swelling of the hands and ankles, shortness of breath, or sudden onset of heart failure;
- effects on liver enzymes.
Before prescribing any medications, doctors discuss their potential side effects with patients. Some biological agents are associated with a higher risk of infection than other drugs in their group.