- Home /
- Branches /
- Rheumatoid arthritis
The Clinical Hospital on Yauza provides reliable diagnosis and effective treatment of rheumatoid arthritis (RA). It is possible to comprehensively conduct all the necessary studies - radiography, magnetic resonance or computed tomography using expert-class equipment using digital technologies, joint puncture and examination of the resulting fluid. Based on the examination results, the rheumatologist prescribes individually selected therapy, consisting of medications and physical procedures. With timely treatment, the condition of most patients improves significantly: the period of stiffness decreases, pain is leveled, and swelling disappears. The hospital’s specialists also offer an innovative method of treating RA—extracorporeal hemocorrection (EG). The use of EG as a component of conservative treatment allows achieving remission in 80-90% of cases.
About the development of pathology
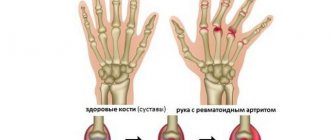
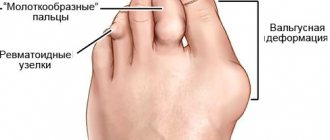
Rheumatoid arthritis is an autoimmune disease that causes inflammation, swelling, pain, and deformation in the joints (mainly the small joints of the hands and feet), leading to impaired mobility. The exact cause of the development of the pathology is still unknown, but scientists are inclined to a multifactorial theory, in which heredity plays a leading role. RA also contributes to:
- frequent infections (including herpes virus);
- toxic effects of medications or environmental factors;
- nervous tension;
- exposure to low temperatures;
- changes in hormonal levels (menopause, pregnancy).
It is assumed that under the influence of toxic substances or under stress, the immune system malfunctions, and its cells begin to attack the tissues of their own body, destroying them.
Currently, rheumatoid arthritis is, along with gout, the most common inflammatory disease of the joints.
Reactive arthritis, causes and symptoms
This type of arthritis is also called Reiter's disease. From the name itself it is clear that the disease is a response to other negative health factors. That is, the infection that causes joint disease primarily affects another organ. Usually this is the genitourinary or digestive system.
With reactive arthritis, the genitourinary organs are first affected, then problems arise with the skin, eyes, and only then the complication spreads to the joints. Patients may experience problems with urination, suffer from ulcers appearing in the genital area, sores on the palms and soles, and a burning sensation in the eyes. Reactive arthritis can be triggered by certain intestinal bacteria. In parallel with reactive arthritis, the patient is usually diagnosed with genitourinary diseases such as urethritis, cystitis, prostatitis or conjunctivitis.
Treatment of chlamydial infection found in the genitourinary system should begin immediately so that reactive arthritis does not have to be treated later.
Better yet, take preventive measures. Reactive arthritis affects several joints at once. But mainly the lower extremities are affected. Along with the joints, the tendons at their attachment to the bone are also often affected. The pain intensifies at night and in the morning, the joints become swollen and red.
Those at risk for rheumatoid arthritis are primarily teenage boys and young men.
The main goal of treating reactive arthritis is to destroy the primary causative agent of the disease. Only by eliminating the source of infection can reactive arthritis be effectively cured. With a long-term and comprehensive approach, a favorable outcome of the disease is possible, up to the complete elimination of pain and inflammation in the joints.
Clinical picture
The disease has several variants of its course. Both an acute onset and a slow gradual onset of symptoms are possible. In the second case, the disease begins with nonspecific manifestations: general weakness, increased body temperature, muscle pain. Subsequently, stiffness and pain in the joints appear. With this course, only one or several joints may be affected, with the gradual involvement of new parts of the musculoskeletal system in the process.
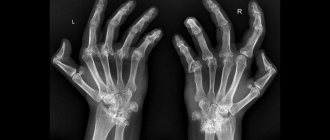
In the classic progression of RA, the disease begins with swelling, pain, and stiffness in the small joints of the hands. The process is symmetrical. A characteristic symptom is increased pain at rest and a decrease in pain with movement. Stiffness is greatest in the morning; its duration reaches several hours.
The danger of untimely treatment of rheumatoid arthritis lies in possible complications both from the musculoskeletal system (deformations and permanent loss of function) and from other internal organs (heart, blood vessels, lungs, kidneys, eyes).
Diagnosis and therapy of rheumatoid arthritis
At the Yauza Clinical Hospital, rheumatoid arthritis is treated by experienced rheumatologists, and if necessary, related specialists are involved (ophthalmologist, cardiologist, neurologist).
Diagnosis of the disease includes: examination by a doctor, general blood test, detailed biochemical and immunological blood test (determining the level of rheumatoid factor, C-reactive protein, antibodies to citrullinated proteins, etc.), radiation methods (radiography, CT or MRI of joints); In some cases, patients are prescribed a microscopic examination of synovial fluid and a biopsy of joint tissue. The set of diagnostic procedures is determined individually, based on the patient’s condition and his wishes.
Treatment of rheumatoid arthritis involves the prescription of anti-inflammatory drugs of various pharmacological groups, the so-called “basic” disease-modifying drugs, physiotherapeutic methods of treatment; in the presence of irreversible joint deformities, surgical treatment is indicated.
Also, extracorporeal hemocorrection (EG, gravitational blood surgery, GCH) has a significant therapeutic effect, especially in the initial stages of the disease and during exacerbation.
Extracorporeal hemocorrection in the treatment of RA
EG methods, as a component of conservative therapy, are indicated for:
- High activity of the process, which is confirmed by laboratory tests;
- Damage to other organs and systems (pleura, kidneys);
- Insufficient effectiveness of standard therapy;
- Severe complications of conservative therapy (peptic ulcer, bleeding disorders, fat and carbohydrate metabolism, etc.);
- The presence of contraindications to the prescription of hormonal therapy.
At the Yauza Clinical Hospital, the following EG methods are used to treat rheumatoid arthritis:
- Combination of cryoapheresis and cell mass incubation. With the help of low temperatures, harmful components (autoantibodies, circulating immune complexes - CIC, pro-inflammatory cytokines) are precipitated and removed from the patient's blood plasma, thereby inhibiting the development of the disease. Isolating cellular elements from the blood and saturating them with a medicinal substance allows us to deliver the medicine in containers exactly to the area of autoimmune inflammation, ensuring its high concentration where it is needed, and significantly reducing the overall effect on the body (leveling side effects).
- Granulocytopheresis. Granulocytes are a type of leukocytes (neutrophils), which, in rheumatoid arthritis, support the activity of inflammatory processes in the tissues of the body, in particular in the joints. Their elimination from the blood contributes to the rapid relief of pain and the onset of remission.
- Cascade plasma filtration. The patient's plasma is passed through a special filter, in which components that trigger and maintain inflammation in tissues (autoantibodies, CEC, complement components, cytokines) are removed from it. This technique allows you to process from 3 to 6 liters of plasma in one session, depriving it of auto-aggressive immune products.
- Lymphocytapheresis. This technique allows you to remove from the blood auto-aggressive blood cells (lymphocytes) that damage body cells (T-lymphocytes), as well as those that produce autoantibodies (B-lymphocytes) that attack your own tissues, which helps to attenuate inflammation processes, reduce doses of immune-suppressing drugs or completely cancellation, increasing the period of remission, slowing down or stopping the patient’s disability and long-term preservation of working capacity.
- Photopheresis. Lymphocytes are isolated from the blood, treated with photosensitizers (drugs that increase the sensitivity of blood cells to the effects of ultraviolet radiation) and subjected to ultraviolet irradiation. Treated lymphocytes stop attacking their own cells and tissues, and inflammatory activity quickly subsides.
Each of the techniques can be used either alone or in combination with other EG techniques. Thus, in case of autoimmune diseases, in particular rheumatoid arthritis, it is possible to diversify the pathogenesis of the disease: reduce the auto-aggressive behavior of cells of the immune system, as well as remove from the blood products already produced by lymphocytes that damage one’s own tissues and organs. Our experienced doctors choose EG methods, taking into account the individual characteristics of each patient and clinical case.
Efficiency of extracorporeal hemocorrection
If you contact the specialists of the Clinical Hospital on Yauza in a timely manner and use GCC techniques, you will not only notice an improvement in your general condition, but also confirm the positive dynamics with objective data. During treatment the following occurs:
- Restoring normal blood counts, reducing the level of autoantibodies, circulating immune complexes, and complement components.
- A significant slowdown in the rate or cessation of autoantibody formation.
- Improving the data of instrumental methods for studying joints (MRI, radiography, CT, ultrasound).
- Rapid onset of remission and increase in its duration by 2.5-3 times.
- Lower intensity of inflammatory processes during subsequent exacerbations of the disease.
- Reducing doses of medications (reducing their side effects) along with increasing their effectiveness.
- In some cases, there is no need to prescribe hormonal therapy.
- Increasing physical activity and reducing the risk of patient disability.
However, it must be remembered that EG methods are not a panacea, but a highly effective method of treating autoimmune pathology, which can significantly slow down the rate of disease development and maintain the maximum possible physical activity of patients with RA. The optimal treatment program is recommended by a rheumatologist based on the results of a comprehensive examination of the patient.
Cost of services
You can see
prices for services
The role of rehabilitation and hardware physiotherapy in the treatment strategy for rheumatic diseases
D.E. Karateev, GBUZ MO "Moscow Regional Research Clinical Institute named after. M.F. Vladimirsky" (MONIKI), Moscow, Russia
E.L. Luchikhina, Moscow Regional Research Clinical Institute named after. M.F. Vladimirsky" (MONIKI), Moscow, Russia
I.P. Osnovina, Federal State Budgetary Educational Institution of Higher Education "Ivanovo State Medical Academy" of the Ministry of Health of Russia, Ivanovo, Russia
A.V. Makevnina, GBUZ MO "Moscow Regional Research Clinical Institute named after. M.F. Vladimirsky" (MONIKI), Moscow, Russia
Summary
Rheumatic diseases represent a serious medical and social problem. The variability of the development mechanisms of this group of diseases requires different approaches: drug and non-drug therapy strategies in modern rheumatology are designed to complement each other. The EULAR recommendations pay insufficient attention to the use of non-drug treatments for joint diseases. According to modern research, multidisciplinary rehabilitation strategies have demonstrated the greatest effectiveness, including, in addition to drug therapy, educational programs, physical training of varying intensity, and the use of hardware rehabilitation methods. Hardware physiotherapy seems to be optimal for the treatment of patients with rheumatic diseases due to greater accessibility and lower cost compared to classical balneo- and peloidotherapy. When using magnetic therapy, there is also greater safety and fewer contraindications to the procedures. The most justified method for treating joint diseases is the use of pulsed magnetic fields, since the sensitivity of biological tissues to them is the highest. The clinical effectiveness of pulsed magnetic therapy has been demonstrated in several randomized, placebo-controlled, multicenter studies. During magnetic field therapy, patients showed a statistically significant reduction in pain and stiffness, and improvement in joint function, which indicates the advisability of including the physical factor in the treatment strategy for rheumatic diseases in combination with drug therapy.
Keywords:
rheumatic diseases, pulsed magnetic field, magnetic therapy, rehabilitation of rheumatic diseases, hardware physiotherapy, non-drug therapy.
Information about the authors:
Karateev D.E - https://orcid.org/0000-0002-2352-4080;
e-mail Luchikhina E.L. — https://orcid.org/0000-0002-6519-1106
Osnovina I.P. — https://orcid.org/0000-0002-4828-5645
Makevnina A.V. — email
Rheumatic diseases (RD), such as osteoarthritis (OA), rheumatoid arthritis (RA), ankylosing spondylitis (AS), psoriatic arthritis (PsA) represent a serious medical and social problem. They are one of the main causes of chronic pain and, accordingly, the widespread use of analgesics and nonsteroidal anti-inflammatory drugs (NSAIDs). These diseases can quickly lead the patient to disability and early death. At the same time, drug therapy for RD requires careful safety monitoring and is very expensive. The variability of the clinical picture, reflecting the individual characteristics of the mechanisms of disease development, requires different approaches. Thus, in the treatment of pain syndromes in rheumatology with the “mechanical” phenotype of musculoskeletal pain, in addition to the use of painkillers (paracetamol, NSAIDs), active correction of biomechanical disorders using orthotics and other rehabilitation methods is indicated [1]. There is no doubt that the influence of drugs and physical factors on the pathogenesis is an example of a complex influence on the pathological process and, with the right approach, can not only expand the arsenal of doctors in the fight against the disease, but also provide additional clinical effect. An example of a successful combination of physical treatment and drug therapy in the field of immunoinflammatory diseases is PUVA therapy for psoriasis, which is included in clinical guidelines, i.e. into the patient management strategy.
Strategy of drug and non-drug therapy in modern rheumatology
The idea of including various rehabilitation technologies in the complex of treatment of RD is absolutely not new and seems completely natural. If we take RA as an example as a classical model disease, then the main directions of treatment for this pathology, from the point of view of a rheumatologist, should be:
- monitoring disease activity
- symptom control (pain syndrome)
- restoration of joint function (including postoperative rehabilitation)
- control of comorbid conditions (osteoporosis, sarcopenia, etc.)
These tasks largely coincide with the tasks of drug therapy, so drug and non-drug treatment methods, in fact, can be used together, potentiating each other’s effects. Unfortunately, at the turn of the second millennium AD in rheumatology, one can observe a disappointing picture of an ever-increasing gap between drug therapy and non-drug treatment methods. In most cases, both in scientific publications and in practice, pharmacotherapy and physiotherapy/rehabilitation methods are used for the same conditions not only by different specialists, but also completely independently of each other, with minimal consideration of interactions. The gap between rheumatology as a science that studies methods of influencing the immunopathological processes of inflammation, and rehabilitation/physiotherapy is currently very wide. This process seems completely illogical, since, on the one hand, in our country rheumatology began to develop rapidly thanks to the activities of the outstanding doctor, scientist and statesman A.I. Nestrov and actually left physiotherapy and balneology [2], and on the other hand, physical methods of treatment are widely used and preferred by many patients, despite all the efforts of adherents of evidence-based medicine.
In recent years, a fairly definite tradition has been established in global rheumatology: when discussing treatment strategies, focus almost exclusively on drug therapy, especially when it comes to systemic immunoinflammatory diseases. A good example in this case are the recommendations for the management of the most significant RDs developed by the European League Against Rheumatism (EULAR) - one of the most authoritative international organizations of rheumatologists. In the 2021 EULAR recommendations for the management of patients with RA, physical therapy methods are mentioned once in the general sense of the possibility of their use [3], and in the updated version of 2021, the terms “physical therapy” and “rehabilitation” are not used at all meet [4]. In the EULAR recommendations for the management of patients with PsA, non-pharmacological methods are also mentioned once: “...optimal management of patients with PsA also requires the use of non-pharmacological methods, such as patient education and regular exercise” [5]. Even the 2021 recommendations for the treatment of hand OA, although they talk about the participation of a physiotherapist in a multidisciplinary team of specialists, only physical exercises appear among specific techniques [6]. Non-pharmacological therapy is presented somewhat more fully in the EULAR guidelines for the management of patients with early arthritis, which indicate the parallel use of patient education programs, dynamic exercises and occupational therapy at the initial stage of drug therapy [7].
Research in the field of integrated management of patients with rheumatic diseases. Motor rehabilitation programs
Recommendations have been published for the use of a number of non-drug treatment methods in patients with RD, which should be used in combination with pharmacotherapy. Thus, clinical guidelines for healthcare workers on the management of patients with chronic rheumatic pain [8] consider several techniques.
According to the “strength” of recommendations, the main methods are divided as follows:
- A (the “strongest” recommendations) - training, physical exercise, orthotics, social rehabilitation, weight control
- B - sleep correction
- D (the “weakest”) - special assessment of pain and function, development of a personalized pain correction plan, creation of a multidisciplinary team
The 2021 EULAR recommendations for physical activity in patients with arthritis [9] state that exercise should be part of standard treatment, prescribed by medical staff in accordance with the plan, taking into account contraindications and characteristics of the patient, should be personalized and adapted to the specific case, without discussing specific techniques.
A significant amount of work is limited to exercise therapy (PT) programs, which is probably due to good tolerance, the absence of contraindications in most patients and the low cost of such treatment. A group of Canadian experts analyzed the effect of physical exercise on pain and function in gonarthrosis [10]. The authors' selection of 26 high-quality studies showed that various strengthening exercise programs with or without other forms of therapeutic exercise generally increased the effectiveness of treatment of knee OA over a 6-month period. Resistance exercise programs have demonstrated significant improvements in pain relief, physical function, and quality of life. The authors concluded that it is necessary to develop combined behavioral and muscle strengthening strategies to maintain the long-term effects of regular exercise programs. According to M. Williams et al. [11], the use of the SARAH specialized exercise program in 490 patients with RA in combination with standard pharmacotherapy for 12 months led to a decrease in the overall pain score, improved performance on quality of life questionnaires, and better dynamics of hand strength in the main group compared to the control.
An impressive result was demonstrated in a study by J. Veldhuijzen van Zanten et al. [12]. These authors compared 2 small groups of RA patients: group 1 (20 patients) received standard therapy + exercises; Group 2 (23 patients) - standard therapy + biological therapy with tumor necrosis factor inhibitors (TNF-i). Patients in group 2, as would be expected, had a significantly better outcome in terms of pain and disease activity. At the same time, equal results were observed in both groups in terms of function and fatigue, while in group 1, in contrast to group 2, positive dynamics of cardiovascular risk and vascular dysfunction were noted. On the other hand, a Cochrane review of exercise programs for AS found only a small reduction in pain and a slight improvement in functional outcomes, with predominantly low-quality evidence from the studies [13].
A similar review of exercise in hand RA [14], which included seven studies involving 841 patients (aged 20 to 94 years), was unable to determine whether exercise improved hand function and pain in the short term. perspective. Exercise appears to have a positive effect on hand function, but at a very small level, and has little or no effect on pain in the medium to long term. It is unclear whether hand clenching exercise improves function at short-term follow-up, although it is also likely to produce modest improvements in the medium to long term.
The work of Danish scientists was recently published [15], who, against the background of modern antirheumatic therapy, used an additional comprehensive program for hand restoration in RA, which included the following components:
1) defensive strategy; 2) auxiliary devices; 3) special methods of using objects in everyday life; 4) strength exercises.
After an 8-week course, patients with RA showed no differences from the control group in assessing pain during movement and at rest, hand strength, or the need to take analgesics. The use of a specialized HEPA program [16], which included strength exercises, aerobic exercises and group exercises, during two years of observation led to a decrease in the overall subjective assessment of pain in patients with RA, but pain sensitivity to palpation and neuropathic pain manifestations in patients remained at the same level.
Thus, complex rehabilitation programs based on exercise therapy in different patient populations gave different results, which indicates the need for a multidisciplinary strategy, including the use of additional technologies, such as balneotherapy and instrumental physiotherapy.
In a large-scale domestic work, E.V. Orlova et al. [17, 18] in 135 patients with early RA against the background of modern drug treatment regimens using synthetic basic drugs and glucocorticoids, 4 rehabilitation schemes were used, based on an educational program and exercise therapy, including high-intensity dynamic training using simulators. Two of them also included local air cryotherapy, one included orthotics. Patients in the control group received only drug treatment. As a result of a 6-month observation, it was demonstrated that a comprehensive program using physiotherapy and orthotics was the best in terms of developing positive dynamics of pain syndrome, increasing functional status, quality of life and locomotor function of the musculoskeletal system, as well as the only rehabilitation technique studied that increased the effectiveness of drug therapy in controlling disease activity according to the standard index. A significant improvement in the medium-term outcomes of early RA under the influence of a comprehensive rehabilitation program, including all the main elements of multidisciplinary medical care, showed the feasibility of introducing rehabilitation technologies into the “Treat to Target” strategy for RA from the moment of diagnosis together with drug therapy [19] .
The place of hardware physiotherapy in the treatment strategy for patients with rheumatic diseases
Hardware physiotherapy seems to be optimal for the treatment of patients with RD due to greater accessibility and lower cost compared to balneotherapy.
At the same time, if we talk about such a common method of hardware physiotherapy as magnetic therapy, then it is also necessary to note greater safety and fewer contraindications for such procedures, which is important, given the scale of the problem of RD in our country. From the standpoint of clinical effectiveness, the most justified is the use of pulsed magnetic fields (PMF), since the sensitivity of biological tissues to them is the highest [20]. Equipment for the treatment of UTIs is widely available in Russia. Portable devices Almag+ and Almag-01 are used for a wide range of pathologies (RH, cardiovascular pathology, neurological conditions, trophic disorders of various origins and severity, etc.). The biophysical effect of magnetic fields is based on the ability to induce electric currents in the affected area, the density of which depends on the rate of change and magnitude of the magnetic field, as well as on the electrical conductivity of biological tissues exposed to the influence of the magnetic field. UTIs cause reversible structural changes in cell membranes and their permeability. There is evidence of the direct effect of IMPs on electrolytes, in particular on Ca2+ ions, which affects cell metabolism, free radical reactions, as well as the ability to have a magnetodynamic effect [20]. The clinical effect of UTI has been repeatedly demonstrated in many studies. Thus, in 50 patients with nonspecific low back pain [21], UTI was used in comparison with the use of a placebo procedure. At the same time, the addition of IMP to the usual physiotherapy protocol in the main group gave a significant effect of reducing the severity of pain and restoring functional ability, more significant than in the control group. A randomized study of the effectiveness of magnetic therapy for gonarthrosis [22] involved 66 patients. After one month of use, PMI induced a significant reduction in visual analogue scale (VAS) pain as well as WOMAC scores compared with placebo; 26% of patients in the UTI group stopped taking NSAIDs/analgesics. No side effects were found. A recent meta-analysis of UTI in knee OA and hand OA [23] found that the UTI group had greater pain relief than placebo in knee OA (standardized mean difference (SMD) -0.54, 95% CI –1.04 to –0.04, p=0.03) and hand OA (SMD= –2.85, 95% CI –3.65 to –2.04, p<0.00001).
Similarly, compared with the placebo procedure, a significant improvement in function was observed in the UTI group in patients with knee and hand OA (SMD = -0.34, 95% CI -0.53 to -0.14, p=0. 0006 and SMD= –1.49, 95% CI –2.12 to –0.86, p<0.00001, respectively).
Sensitivity analysis showed that exposure durations of up to 30 min per procedure had better effects compared to exposure durations of more than 30 min. Three meta-analysis studies reported adverse events, however, the pooled results revealed that there were no significant differences in safety between the UTI and placebo treatment groups. The latest large study of UTI in OA [24] was published in 2020. The study group consisted of 231 patients with knee OA (77.9% women, mean age 61.9±12.2 years, body mass index 30.6 ±5.8 kg/m2, median disease duration - 5.0 [2.0; 10.0] years). The patients were randomized into two groups.
Patients of the 1st group underwent IMP using the ALMAG+ device for 14 days, patients of the 2nd group received a false IMP (a device that completely imitates the ALMAG+ device, but does not create a magnetic field). The dynamics of the WOMAC index, the severity of pain at rest and during movement on a 100-mm VAS, the need for NSAIDs and the severity of improvement from the patients’ point of view (on a 5-point scale) were assessed. During the therapy, a statistically significant decrease in pain and stiffness and improvement in function were noted. Thus, the median WOMAC pain index in group 1 decreased from 231 [180; 290] to 110 [60; 166.3] (p<0.001); in group 2 - from 212.4 [145; 260] to 143 [76.5; 200] (p<0.001), the severity of pain at rest (according to VAS) decreased in group 1 from 47 [27.8; 60] to 20 [10; 30] mm (p<0.001); in group 2 - from 40 [20;57.5] to 20 [7.5; 40] mm (p<0.001).
During the therapy, the need for taking NSAIDs also decreased: in group 1, the drug was discontinued or its dosage was reduced in 33.1% of patients, in group 2 - in 16.8% (p = 0.006). For all indicators, the dynamics in patients of group 1 were statistically more significant than in group 2. The treatment result was rated as “good” or “excellent” by 58.5% of patients in group 1 and 39.8% in group 2 (p<0.001). No serious adverse reactions were observed during true or sham UTI therapy. In two patients receiving sham UTI therapy, treatment was interrupted due to increased joint pain. Currently, a long-term, double-blind, placebo-controlled trial “Evaluation of the effectiveness and safety of the magnetic therapy device “ALMAG+” in the treatment of osteoarthritis of the knee joints” of the portable therapeutic device “ALMAG+” is being conducted (Certificate EN ISO 13485: 2012 + AC: 2012, registration No. 44221 117836, Reg. No. 3007075140).
Patients with primary and secondary (as a manifestation of inflammatory rheumatic disease) OA of the knee joint of Kellgren-Lawrence stage I-III were included in the study against the background of stable drug therapy. Three UTI courses of 20 procedures were planned over the course of a year in the active treatment group and the placebo group (sham therapy - inactive device), 35 patients each. In addition to clinical methods for assessing effectiveness, instrumental monitoring is carried out using ultrasound and MRI of the knee joint. The study protocol was approved by the local ethics committee.
Preliminary results of this ongoing study were reported at the EULAR 2021 Congress [25]. To date, 23 patients (7 men, 16 women, mean age 54.6±11.2 years) have completed the 1st course of UTI. The table presents the differences (Δ) between the initial indicators and the indicators in patients with knee OA after the 1st course of UTI for the main clinical parameters in the active treatment and placebo (sham procedures) groups.
There was a significantly more pronounced change in pain at rest, as well as a trend towards more pronounced changes in pain on movement and in the WOMAC index and Lequesne index in the group receiving active procedures. No treatment-related adverse events were observed.
Dynamics of decrease (Δ) in the main clinical parameters in patients with knee OA after the 1st course of UTI [25]
| Parameter | Active device (n=11) | Placebo - inactive device (n=12) | p |
| Δ VAS pain with movement Δ VAS pain at rest Δ WOMAC index Δ Lequesne index [26] | 15 [0; 38,5] 10 [0; 34] 3 [2; 10] 3 [0; 4] | 5 [1,25; 10,5] 1 [0; 2,75] 2 [0; 4,5] 1 [0,25; 2,5] | 0,053 0,043 0,174 0,258 |
Note. p — significance of differences between groups; VAS - visual analogue scale; WOMAC—Western Ontario and McMaster Universities Osteoarthritis Index [27].
Conclusion
Thus, the use of hardware treatment methods in the complex treatment strategy for RD is pathogenetically justified and clinically effective. Physiotherapeutic treatment of UTIs using the ALMAG+ device has a fairly pronounced analgesic and mobilizing effect, which, if widely used, can lead to the following positive trends:
- better pain control;
- reducing the need for analgesics and NSAIDs;
- increasing mobility;
- improving quality of life.
Treatment of UTI is relatively safe compared to other types of physiotherapy and balneotherapy, and is also more accessible. All of the above indicates the advisability of including hardware treatment methods, including UTI, in the treatment strategy for OA and RA, as well as, possibly, other RDs in in combination with drug therapy.
Literature:
- Karateev A.E. Musculoskeletal pain: identification of clinical phenotypes and a rational approach to treatment. Almanac of Clinical Medicine. 2019;47(5):445-453.
- Shostak N.A. School of Academician A.I. Nesterova. Medical Affairs. 2006;3:86-90.
- Smolen JS, Landewé R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, Nam J, Ramiro S, Voshaar M, van Vollenhoven R, Aletaha D, Aringer M, Boers M, Buckley CD, Buttgereit F, Bykerk V, Cardiel M, Combe B, Cutolo M, van Eijk-Hustings Y, Emery P, Finckh A, Gabay C, Gomez-Reino J, Gossec L, Gottenberg JE, Hazes JMW, Huizinga T, Jani M, Karateev D, Kouloumas M, Kvien T, Li Z, Mariette X, McInnes I, Mysler E, Nash P, Pavelka K, Poór G, Richez C, van Riel P, Rubbert-Roth A, Saag K, da Silva J, Stamm T, Takeuchi T, Westhovens R , de Wit M, van der Heijde D. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76:960977. https://doi.org/:10.1136/annrheumdis-2016-210715
- Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, McInnes IB, Sepriano A, van Vollenhoven RF, de Wit M, Aletaha D, Aringer M, Askling J, Balsa A, Boers M, den Broeder AA , Buch MH, Buttgereit F, Caporali R, Cardiel MH, Cock DD, Codreanu C, Cutolo M, Edwards CJ, van Eijk-Hustings Y, Emery P, Finckh A, Gossec L, Gottenberg JE, Hetland ML, Huizinga TWJ, Koloumas M, Li Z, Mariette X, Müller-Ladner U, Mysler EF, da Silva JAP, Poor G, Pope JE, Rubbert-Roth A, Ruyssen-Witrand A, Saag KG, Strangfeld A, Takeuchi T, Voshaar M, Westhovens R , van der Heijde D. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2021 update. Ann Rheum Dis. 2020;79:685-699. https://doi.org/10.1136/annrheumdis-2019-216655
- Gossec L, Baraliakos X, Kerschbaumeret A, de Wit M, McInnes I, Dougados M, Primdahl J, McGonagle DG, Aletaha D, Balanescu A, Balint PV, Bertheussen H, Boehncke WH, Burmester GR, Canete JD, Damjanov NS, Kragstrup TW, Kvien TK, Landewé RBM, Lories RJU, Marzo-Ortega H, Poddubnyy D, Manica SAR, Schett G, Veale DJ, den Bosch FEV, van der Heijde D, Smolen JS. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2021 update. Ann Rheum Dis. 2020;79:700-712. https://doi.org/10.1136/annrheumdis-2020-217159
- Kloppenburg M, Kroon FPB, Blanco FJ, Doherty M, Dziedzic KS, Greibrokk E, Haugen IK, Herrero-Beaumont G, Jonsson H, Kjeken I, Maheu E, Ramonda R, Ritt MJPF, Smeets W, Smolen JS, Stamm TA, Szekanecz Z, Wittoek R, Carmona L. 2021 update of the EULAR recommendations for the management of hand osteoarthritis. Ann Rheum Dis. 2019;78:16-24. https://doi.org/10.1136/annrheumdis-2018-213826
- Combe B, Landewe R, Daien CI, Hua C, Aletaha D, Álvaro-Gracia JM, Bakkers M, Brodin N, Burmester GR, Codreanu C, Conway R, Dougados M, Emery P, Ferraccioli G, Fonseca J, Raza K, Silva-Fernández L, Smolen JS, Skingle D, Szekanecz Z, Kvien TK, van der Helm-van Mil A, van Vollenhoven R. 2021 update of the EULAR recommendations for the management of early arthritis. Ann Rheum Dis. 2017;76(6):948-959. https://doi.org/10.1136/annrheumdis-2016-210602
- Geenen R, Overman CL, Christensen R, Åsenlöf P, Capela S, Huisinga KL, Husebø MEP, Köke AJA, Paskins Z, Pitsillidou IA, Savel C, Austin J, Hassett AL, Severijns G, Stoffer-Marx M, Vlaeyen JWS, Fernández-de-Las-Peñas C, Ryan SJ, Bergman S. EULAR recommendations for the health professional's approach to pain management in inflammatory arthritis and osteoarthritis. Ann Rheum Dis. 2018;77(6):797-807. https://doi.org/10.1136/annrheumdis-2017-212662
- Rausch Osthoff AK, Niedermann K, Braun J, Adams J, Brodin N, Dagfinrud H, Duruoz T, Esbensen BA, Günther KP, Hurkmans E, Juhl CB, Kennedy N, Kiltz U, Knittle K, Nurmohamed M, Pais S, Severijns G, Swinnen TW, Pitsillidou IA, Warburton L, Yankov Z, Vlieland TPMV. 2021 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann Rheum Dis. 2018;77(9):1251-1260. https://doi.org/10.1136/annrheumdis-2018-213585
- Brosseau L, Taki J, Desjardins B, Thevenot O, Fransen M, Wells GA, Imoto AM, Toupin-April K, Westby M, Gallardo ICA, Gifford W, Laferrière L, Rahman P, Loew L, Angelis GD, Cavallo S, Shallwani SM, Aburub A, Bennell KL, der Esch MV, Simic M, McConnell S, Harmer A, Kenny GP, Paterson G, Regnaux JP, Lefevre-Colau MM, McLean L. The Ottawa panel clinical practice guidelines for the management of knee osteoarthritis. Part two: strengthening exercise programs. Clin Rehabil. 2017;31(5):596-611. https://doi.org/10.1177/0269215517691084
- Williams MA, Williamson EM, Heine PJ, Nichols V, Glover MJ, Dritsaki M, Adams J, Dosanjh S, Underwood M, Rahman A, McConkey C, Lord J, Lamb SE. Strengthening And stretching for Rheumatoid Arthritis of the Hand (SARAH). A randomized controlled trial and economic evaluation. Health Technol Assess. 2015;19(19):1-222. https://doi.org/10.3310/hta19190
- Veldhuijzen van Zanten JJCS, Sandoo A, Metsios GS, Stavropoulos-Kalinoglou A, Ntoumanis N, Kitas GD. Comparison of the effects of exercise and anti-TNF treatment on cardiovascular health in rheumatoid arthritis: results from two controlled trials. Rheumatol Int. 2019;39(2):219-225. https://doi.org/10.1007/s00296-018-4183-1
- Regnaux JP, Davergne T, Palazzo C, Roren A, Rannou F, Boutron I, Lefevre-Colau MM. Exercise programs for ankylosing spondylitis. Cochrane Database Syst Rev. 2019;10(10):CD011321. . https://doi.org/10.1002/14651858.CD011321.pub2
- Williams MA, Srikesavan C, Heine PJ, Bruce J, Brosseau L, Hoxey-Thomas N, Lamb SE. Exercise for rheumatoid arthritis of the hand. Cochrane Database Syst Rev. 2018;7(7):CD003832. https://doi.org/10.1002/14651858.CD003832.pub3
- Ellegaard K, von Bülow C, Røpke A, Bartholdy C, Hansen IS, Rifbjerg-Madsen S, Henriksen M, Wæhrens EE. Hand exercise for women with rheumatoid arthritis and decreased hand function: an exploratory randomized controlled trial. Arthritis Res Ther. 2019;21(1):158. https://doi.org/10.1186/s13075-019-1924-9
- Löfgren M, Opava CH, Demmelmaier I, Fridén C, Lundberg IE, Nordgren B, Kosek E. Long-term, health-enhancing physical activity is associated with reduction of pain but not pain sensitivity or improved exercise-induced hypoalgesia in persons with rheumatoid arthritis. Arthritis Res Ther. 2018;20(1):262. https://doi.org/10.1186/s13075-018-1758-x
- Orlova E.V., Karateev D.E., Amirdzhanova V.N. The effectiveness of an individual rehabilitation program for patients with rheumatoid arthritis. Scientific and practical rheumatol. 2012;50(1):45-53.
- Orlova E.V., Karateev D.E., Kochetkov A.V. Comprehensive rehabilitation of patients with early rheumatoid arthritis: results of a 6-month program. Scientific and practical rheumatol. 2013;51(4):398-406.
- Orlova E.V., Karateev D.E., Kochetkov A.V., Surnov A.V. Comparative effectiveness of four rehabilitation programs in patients with early rheumatoid arthritis. Bulletin of the Ivanovo Medical Academy. 2014;19(2):37-42.
- Agafonov B.V., Sekirin A.B., Smirnova S.N., Topchiy N.V., Gorenkov R.V., Larinsky N.E., Sushinsky V.E., Ivanitsky L.V. The use of magnetic therapy and some other therapeutic physical factors in general medical practice (family medicine). A manual for doctors. M: MONIKI; 2021.
- Elshiwi AM, Hamada HA, Mosaad D, Ragab IMA, Koura GM, Alrawaili SM. Effect of pulsed electromagnetic field on nonspecific low backpain patients: a randomized controlled trial. Braz J Phys Ther. 2019;23(3):244-249. https://doi.org/10.1016/j.bjpt.2018.08.004
- Bagnato G, Miceli G, Marino N, Sciortino D, Bagnato GF. Pulsed electromagnetic fields in knee osteoarthritis: a double blind, placebo-controlled, randomized clinical trial. Rheumatology. 2016;55(4):755-762, https://doi.org/10.1093/rheumatology/kev426
- Wu Z, Ding X, Lei G, Zeng C, Wei J, Li J, Li H, Yang T, Cui Y, Xiong Y, Wang Y, Xie D. Efficacy and safety of the pulsed electromagnetic field in osteoarthritis: a meta- analysis. BMJ Open. 2018;8(12):e022879. https://doi.org/10.1136/bmjopen-2018-022879
- Karateev A.E., Pogozheva E.Yu., Sukhareva M.L., Lila A.M., Ivanov A.V., Osnovina I.P., Shchashkova O.V., Borisova S.V., Larinsky N. S., Israelyan Yu.A., Afoshin S.A., Bondarenko T.P., Repchanskaya E.A., Chernyavskaya L.A., Pupina S.P., Darmova T.V. Evaluation of the effectiveness and safety of magnetic therapy for osteoarthritis. Results of the multicenter, blind, placebo-controlled study COSMO (Clinical Evaluation of Modern Magnetic Therapy for Osteoarthritis). Scientific and practical rheumatology. 2020;58(1):55-61.
- Karateev D, Makevnina A, Tangieva A, Luchikhina E, Hamhoeva H. Double-blind placebo-controlled trial of pulsed electromagnetic field therapy in knee osteoarthritis (preliminary results). Ann Rheum Dis. 2020;79(suppl1):1918, https://doi.org/10.1136/annrheumdis-2020-eular.6396
- Lequesne MG. The algofunctional indicators for hip and knee osteoarthritis. J Rheumatol. 1997;24:779-781.
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt L. Validation study of WOMAC: a health status instrument for measuring clinically-important patient-relevant outcomes following total hip or knee arthroplasty in osteoarthritis. Journal of Orthopedic Rheumatology. 1988;1:95-108.
Source:
Issues of balneology, physiotherapy and therapeutic physical culture, 2021, T. 97, No. 5, p. 87-93 doi.org/10.17116/kurort20209705187
(PDF 158 KB)