Juvenile arthritis is a common rheumatic disease that affects children under 16 years of age, especially girls. Epidemiological data indicate that juvenile arthritis affects an average of 9 children out of 100,000. It is characterized by high disability, so timely treatment and diagnosis is the primary task of parents at the slightest suspicion of JA.
A cause for concern should be persistent pain that lasts more than 6 weeks. You should consult a doctor already in the 2nd week of ill health. But how to recognize the onset of the disease?
What is juvenile arthritis?
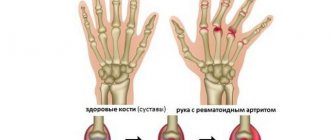
Juvenile, or in other words, juvenile arthritis is a type of rheumatoid and is a pathology whose degenerative-dystrophic processes affect the connective tissues of the human body, having a detrimental effect on the functioning of the joints, heart and kidneys.
Juvenile arthritis is the most common in children and is detected mainly in adolescence and belongs to the category of the most common rheumatological diseases of children. According to statistics, the peak incidence occurs in the period from 10 to 14 years
.
The uncontrolled course of the pathology can cause a weakening of the general condition of the body, as well as a sharp decrease in immune abilities.
Traditional treatment
Alternative medicine helps to cope with pathology in the primary stages of development and is included in general therapeutic procedures. Signs of juvenile arthritis are relieved by:
- Decoction of willow bark - pour a tablespoon of plant material into a small container, pour boiling water over it, and keep on the stove for 20 minutes. After filtration, the medicine is consumed twice a day for one month. It can serve as the basis for lotions and compresses that are applied to sore joints.
- A decoction of bay leaves - a small spoon of pre-crushed raw material, pour a glass of boiling water, cook for 10 minutes. The finished substance is poured into a thermos, kept for 12 hours, consumed a third of a cup three times a day, before meals.
- Honey ointment - 150 ml of natural product mixed with 200 ml of black radish juice, half a glass of vodka, 15 g of table salt. The finished product is rubbed into problem areas until a lasting result is obtained.
A decoction of bay leaves is one of the alternative treatment options.
Important: alternative methods cannot completely replace medications.
Causes of juvenile arthritis
Determining the exact causes of the development of the disease is not easy, because each case is individual and has a number of features of the anamnestic picture.
Research conducted by domestic and foreign scientists has determined that the main factors in the development of juvenile arthritis are hereditary predisposition
, as well as the presence
of infectious agents
and
autoimmune disorders
.
Among the most common causes of the development of juvenile pathology are:
- viral and bacterial pathogens;
- injury to joint tissues;
- systematic hypothermia of the body;
- negative impact of long-term exposure to solar radiation.
It is worth noting that the initiator of the disease can be the active influence of one or more factors in combination with genetic predisposition.
Diagnosis of the disease
The diagnosis of rheumatoid arthritis in children of this type should be differential. To determine the disease, the following research methods are needed:
- Laboratory blood tests that will make it possible to determine the level of ESR and the presence of rheumatoid factor.
- X-ray of the affected joints, which will determine the degree of development of the disease, the condition of bone and cartilage tissue.
- Ultrasound of internal organs.
- Collecting a detailed medical history, which will allow us to establish a hereditary predisposition.
- Fundus examination.
- External examination of the patient with recording of his complaints.
Since juvenile chronic arthritis has nonspecific symptoms, only differential diagnosis can determine it. The effectiveness of treatment largely depends on its quality.
About the features of treating the disease without pills, see the video below:
Symptoms and signs of juvenile arthritis
Juvenile arthritis in children is dangerous because the peculiarities of the course of pathological processes can negatively affect the growth zones and disrupt the process of natural growth of the child
.
The main clinical signs of juvenile arthritis are stiffness, swelling, tenderness and local redness of the skin of the joint.
To the initial signs
Juvenile arthritis may include:
- weakness, general malaise, relatively slight increase in body temperature, loss of appetite, cold sweat;
- rashes of various kinds in the area of the affected joint, not accompanied by itching;
- limited joint mobility in the morning;
- local swelling, pain in one or more large joints;
- periodic heart pain, shortness of breath.
To signs of systemic manifestations
at the stage of the acute form of development of juvenile pathology include:
- heart pain, bluish skin;
- cough, difficulty breathing;
- disorders of the gastrointestinal tract;
- enlarged liver, spleen;
- renal dysfunction;
- inflammation of the visual organs;
- growth slowdown.
If you have one or more symptoms, you should urgently seek qualified medical help.
Classification of the disease
Adolescent, or juvenile, rheumatoid arthritis is a very complex and dangerous disease. In adults it may develop more slowly. Treatment of the pathology should begin immediately - immediately after the patient’s symptoms are described and differential diagnosis is carried out.
Naturally, you should also consider what types of diseases exist:
By type of lesion:
- Articular. This juvenile (youthful) arthritis is characterized by the fact that the main inflammatory process is localized only in the joints, without affecting other structures.
- Systemic. In this case, the pathology additionally extends to the internal organs. This form of rheumatoid arthritis is very severe and dangerous. It often leads to permanent disability.
According to the distribution of the lesion:
- Juvenile oligoarthritis (oligoarticular). It is characterized by the fact that no more than 4 joints are affected in a child. In this case, not only large but also small joints are affected. This type of juvenile rheumatoid arthritis is diagnosed in children over 1 year of age. This form of the disease can also be limited to affecting only a few joints, but in some cases it progresses and spreads.
- Juvenile polyarthritis. Here the pathology affects the upper and lower extremities. The number of diseased joints is more than 5. The neck and jaw joints may also be affected. Most often, such juvenile arthritis occurs in girls. Treatment of the disease is mainly carried out in a hospital.
By rate of progression:
- Slow.
- Moderate.
- Fast.
Learn more about the disease from this video:
According to immunological characteristics:
- Juvenile seronegative rheumatoid arthritis. Its peculiarity is that rheumatoid factor is not detected in the blood.
- Juvenile seropositive rheumatoid arthritis. This type of disease is more severe. However, it can be detected using the presence of a rheumatological marker in the blood.
According to the nature of the flow:
- Reactive (acute). This is a malignant form of the disease that progresses rapidly. The prognosis in this case is unfavorable.
- Subacute. It is characterized by slow development and progression. It usually affects only one side of the body at first. In the future, the pathological process covers other joints. In this case, the prognosis is favorable, since the disease is treatable.
Juvenile rheumatoid arthritis can present in different ways. However, in any case, its treatment is necessary, complex and lifelong.
Stages of development of juvenile arthritis
Juvenile rheumatoid arthritis occurs in 4 stages:
- Initial – lasts up to 6 months
. Damage to articular bone tissue. - Early – lasts from 6 to 12 months
. Destruction of cartilage tissue, proliferation of the joint membrane, reduction of the joint lumen. - Expanded - can last up to two years
. The appearance of erosions on cartilage and bone tissue, the formation of subluxations. - Late – lasts more than 2 years and leads to disability
. Fusion of bone tissue, loss of limb mobility.
How does juvenile rheumatoid arthritis develop?
The disease provokes humoral immunity. The fact is that pathological changes occur in the synovial membrane of the joint, as a result of which blood microcirculation is disrupted and hard tissues are gradually destroyed. In this case, altered immunoglobulins are produced in the affected joints.
The defense system begins to intensively produce antibodies, which attack the body’s own tissues. Because of this, an inflammatory process begins to develop, which is almost impossible to eliminate. It is chronic and is constantly supported by the immune system.
Through the circulatory and lymphatic systems, antigens spread throughout the body, affecting other structures.
Types of juvenile arthritis
Depending on the symptoms, there are such forms of juvenile arthritis as:
- pauciarticular
– occurs between the ages of 1 and 6 years, asymmetrically affects large joints; - seropositive juvenile polyarthritis
- develops in children over 8 years of age, affects joints of various sizes; - seronegative juvenile polyarthritis
- develops up to 15 years of age, characterized by numerous joint lesions (often the temporomandibular and cervical spine); - with systemic onset
– does not depend on gender and age, affects joints, lymph nodes and internal organs; - idiopathic
- etiology unknown.
Symptoms
Symptomatic features depend on the existing pathology; the articular type differs from the system-wide type in its main features. The first option includes:
- involvement of all subgroups of the articular apparatus with severe swelling and edema;
- low temperature in the damaged area;
- discomfort at rest and movement;
- stiffness in the morning – lasts more than 30 minutes;
- problems with flexion function, development of subluxations;
- in the last stages there is a loss of joint function.
Symptoms depend on the stage of development of the pathological process.
The systemic variant anomaly has more pronounced and aggressive symptoms. The main manifestations include:
- elevated temperature - up to 40 degrees;
- with simultaneous inflammation of several or one joint;
- The pathology is characterized by brownish spots near the nail plates;
- problems with the functionality of the heart - discomfort, shortness of breath, arrhythmic abnormalities, fast or slow heartbeat;
- an increase in the volume of the liver, lymph nodes, spleen;
- uveitis.
Important: Local changes in the form of swelling and edema of the joints lead to the conclusion that a chronic course of the disease initially develops, and only after that does rheumatoid juvenile arthritis develop.
Infants are also affected by the pathology; the problem of diagnosis is due to the fact that at this age children do not walk and joint damage is more difficult to determine.
Diagnosis of juvenile arthritis
In order to conduct a thorough examination and make the most accurate diagnosis, it is necessary to contact a surgeon, rheumatologist, traumatologist or therapist, who, in accordance with the existing symptoms, will determine the right specialist.
Diagnosis of juvenile arthritis in children and adults is carried out in two stages:
- laboratory
– includes clinical, biochemical and immunological studies; - instrumental
- the use of X-ray/ultrasound examination, as well as computed tomography and magnetic resonance imaging.
Based on the results of the diagnostic study, a diagnosis is established and the optimal treatment plan is determined, using physiotherapeutic methods and drug therapy.
Diagnostic methods
The appointment is conducted by a rheumatologist who studies the family history and the presence of similar diseases in close relatives. After a visual examination and palpation examination, the doctor clarifies the symptomatic signs, the strength of their severity and the time of formation. Clinical diagnosis of juvenile rheumatoid arthritis allows you to confirm or refute the initial diagnosis.
Laboratory (Analysis)
The patient is sent for delivery:
- a detailed and biochemical study of blood with the determination of C reactive protein, total protein, glucose, creatinine, an indicator of liver enzyme activity;
- general urine analysis, study of biomaterial for rheumatoid factor;
- immunological blood test.
If the history indicates frequent cases of tonsillitis, then an additional blood test is carried out for the presence of streptococcal microorganisms.
Instrumental
The patient undergoes an ultrasound scan of the joints, heart, kidneys, abdominal cavity, X-ray images, and an electrocardiogram. Only after receiving all the information and identifying the peculiarities of the course of the disease, the patient is prescribed an individual therapy program.
Treatment of juvenile arthritis
Treatment of pathology can be carried out both on an outpatient basis and in a hospital.
Inpatient treatment is required in situations where:
- there is a suspicion of the presence of the disease, but the diagnosis has not yet been confirmed;
- the degree of activity in the development of pathological processes reaches a medium/high pace;
- monitoring of the effectiveness/safety of the therapy used is necessary.
In a situation where the disease is detected in early childhood (from 1 to 4 years), hospitalization is mandatory
. This is due to the need to achieve remission as quickly as possible. Otherwise, the child's growth and development may be delayed.
Outpatient treatment is used during periods of remission or low activity of pathological processes.
Physiotherapy as a method of treating juvenile arthritis
Physiotherapeutic measures are carried out in parallel with drug therapy, as well as during the period of remission, to consolidate the achieved results.
The key goal of physiotherapy is to reduce inflammation in the joints
,
reduction of pain
, as well as
activation of metabolic processes within joint tissues
.
Massage as a treatment for juvenile arthritis
Massage is an additional means of combating degenerative processes. The positive effect of the procedure is noticeable at any stage of the disease
.
It is important to note that combining massage with physiotherapeutic procedures is possible only outside the phase of exacerbation of the disease
.
Exercise therapy for juvenile arthritis
Physical activity
in the form
of exercise therapy
for juvenile arthritis - a necessary component of treatment.
A set of exercises should be selected by a specialist, taking into account the stage of development of the pathology, as well as the individual characteristics of the patient.
Neglecting counseling sessions and advice from the attending physician can lead to loss of the beneficial effect of therapy and aggravation of the patient’s condition
.
Juvenile arthritis: possibilities of drug and non-drug treatment at the present stage
Juvenile arthritis (JA) is arthritis of unknown cause, lasting more than 6 weeks, developing in children under 16 years of age. When making a diagnosis, it is necessary to exclude other joint pathologies (see table “Differential diagnosis of juvenile arthritis” on pages 60–61).
JA is one of the most common and most disabling rheumatic diseases found in children. The incidence of JA ranges from 2 to 16 per 100 thousand children under the age of 16 years. The prevalence of JA in different countries ranges from 0.05 to 0.6%. The prevalence of JA in children under 18 years of age in the Russian Federation reaches 62.3, the primary incidence is 16.2 per 100 thousand, including in adolescents the corresponding figures are 116.4 and 28.3, and in children under 14 years of age - 45 .8 and 12.6. Girls are more often affected by rheumatoid arthritis (RA). Mortality rate is within 0.5–1%.
Classification
In the International Classification of Diseases, X Revision (ICD-10), juvenile arthritis is included in category M08:
- M08.0 - youthful (juvenile) rheumatoid arthritis (RF+ and RF–);
- M08.2 - youthful (juvenile) arthritis with systemic onset;
- M08.3 - youthful (juvenile) polyarthritis (seronegative);
- M08.4 - pauciarticular youthful (juvenile) arthritis;
- M08.8 - other juvenile arthritis;
- M08.9 - unspecified juvenile arthritis.
There are three more classifications of the disease: the American College of Rheumatology (AKP) classification of juvenile rheumatoid arthritis (JRA), the European League Against Rheumatism classification of JIA (juvenile chronic arthritis), and the International League of Rheumatological Associations classification of JIA (juvenile idiopathic arthritis) (Table 1). Comparative characteristics of all classification criteria are presented in table. 2.
Treatment
1. Non-drug treatment
Mode
During periods of exacerbation of the disease, the child’s motor activity should be limited. Complete immobilization of joints with the application of splints is contraindicated; this contributes to the development of contractures, atrophy of muscle tissue, aggravation of osteoporosis, and rapid development of ankylosis. Physical exercise helps maintain the functional activity of joints. Cycling, swimming, walking are useful. Running, jumping, active games are undesirable. It is recommended to maintain a straight posture when walking and sitting, and sleep on a hard mattress and a thin pillow. Limit psycho-emotional stress, exposure to the sun.
Diet
Eating foods high in calcium and vitamin D to prevent osteoporosis. In patients with Cushing's syndrome, it is advisable to limit the consumption of carbohydrates and fats; a protein diet is preferable.
Therapeutic exercise (physical therapy)
An essential component of treatment for JA. Daily exercises are necessary to increase the range of motion in the joints, eliminate flexion contractures, and restore muscle mass. If the hip joints are affected, traction procedures on the affected limb after preliminary consultation with an orthopedist, walking on crutches. During the period of development of coxitis and aseptic necrosis of the hip joints, the patient’s movement without crutches is contraindicated. Physical therapy should be carried out in accordance with the individual capabilities of the patient.
Orthopedic correction
Static orthoses such as splints, splints, insoles and dynamic cuts in the form of lightweight removable devices. Static orthoses require intermittent immobilization - they should be worn or put on during free time and must be removed during the day to stimulate the muscular system during exercise, classes, occupational therapy, and the toilet. For severe osteoporosis in the thoracic and lumbar spine - wearing a corset or reclining system; with damage to the joints of the cervical spine - the head holder (soft, hard).
2. Drug treatment
Several groups of drugs are used to treat JA: non-steroidal anti-inflammatory drugs (NSAIDs), glucocorticoids (GCs), immunosuppressants and biological agents obtained by genetic engineering. The use of NSAIDs and GCs helps to quickly reduce pain and inflammation in the joints, improve function, but does not prevent the progression of joint destruction and disability of patients. Immunosuppressive and biological therapy stops the development of destruction and disability of patients.
Glucocorticoids
Pulse therapy
Pulse therapy with GC is carried out in case of the development of severe systemic manifestations of JA (carditis, pneumonitis, polyserositis, hemophagocytic syndrome).
Advantages:
- rapid (within 24 hours) suppression of the activity of the inflammatory process and relief of symptoms of the disease;
- rapid elimination of the drug, short-term suppression of the adrenal glands, restoration of their function after 4 weeks.
Administration scheme:
- the dose of methylprednisolone is 10–20 mg/kg per administration (not higher than 500 mg);
- methylprednisolone is dissolved in 200 ml of 5% glucose solution or 0.9% sodium chloride solution;
- duration of administration 30–40 minutes;
- the drug is administered once a day in the morning;
- GC pulse therapy is carried out for 3–5 consecutive days.
When using GC pulse therapy, adverse events may develop.
Transfusion adverse events:
- increased blood pressure (BP);
- hyperglycemia;
- facial redness;
- headache, dizziness;
- change in taste;
- heartbeat;
- euphoria.
Long-term unjustified use of intravenous GCs causes the development of severe adverse events:
- persistent increase in blood pressure;
- severe steroid osteoporosis. It is most pronounced in the thoracic and lumbar spine. It manifests itself as a decrease in the height of the vertebral bodies, compression fractures. Accompanied by symptoms of compression of the spinal cord roots;
- obesity. Has characteristic features - a moon-shaped face, fat deposition on the neck, chest, abdomen, steroid “hump”, atrophy of the muscles of the arms and legs;
- erosive and ulcerative processes in the upper gastrointestinal tract;
- steroid myopathy;
- posterior capsular cataract;
- skin changes (hypertrichosis, purulent skin infection, stretch marks, skin trauma, rough scars, impaired wound healing, steroid acne on the face and torso).
HA for oral administration
GCs have a rapid anti-inflammatory effect in most patients. High doses of prednisolone (more than 0.6 mg/kg/day) stop acute inflammatory changes in the joints and control the activity of systemic manifestations. However, reducing the dose of prednisolone and its withdrawal, as a rule, lead to an exacerbation of the disease. And re-prescribing prednisolone at the original dose is no longer effective enough for most patients.
In connection with the above, the indication for prescribing oral GC is only the ineffectiveness of intravenous GC, immunosuppressive and biological drugs, in combination or without intravenous GC.
If GC is prescribed orally, the dose of prednisolone should not exceed 0.2–0.5 mg/kg per day, the daily dose is 15 mg.
The maximum dose of GC should be taken no more than a month after achieving remission. Subsequently, the dose of GC is gradually reduced to a maintenance regimen, followed by its abolition. Prednisolone must be prescribed with an adequate dose of methotrexate and/or cyclosporine (see “Treatment of juvenile arthritis with systemic onset”). The reduction in the dose of prednisolone should be slow; a maintenance dose (0.1 mg/kg body weight) should be taken for at least one year.
Tactics for reducing the dose of oral GCs.
The rate of reduction in the dose of GC should depend on its initial daily dose:
- up to 15 mg - reduce by 1.25 mg 1 time every 3-4 days;
- from 15 to 10 mg - reduce by 1.25 mg once every 5-7 days;
- from 10 mg to 5 mg - alternating decrease. On even days the child takes prednisolone at the original dose, on odd days - 1/8 tablet less. This regimen is maintained for 7–10 days. In the absence of withdrawal syndrome, 1/8 tablet can be discontinued. Over the next 7–10 days, the child takes a constant (after stopping 1/8 tablet) dose of prednisolone;
- from 5 mg until complete withdrawal - alternating reduction. On even days the child takes prednisolone at the original dose, on odd days - 1/8 tablet less. This regimen is maintained for 14 days. In the absence of withdrawal syndrome, 1/8 tablet can be discontinued. Over the next 4 weeks, the child takes a constant dose of prednisolone.
Dose reduction and discontinuation of prednisolone are usually accompanied by the development of withdrawal syndrome, especially in patients who have been receiving it for a long time. Withdrawal syndrome is manifested by myalgia, arthralgia, muscle tremors, fever, nausea, vomiting, depression.
For the purpose of replacement therapy for withdrawal syndrome, intravenous administration of methylprednisolone at a dose of 5 mg/kg can be used.
Discontinuation of prednisolone for 2–4 months, prescribed at a dose of 1.0 mg/kg or higher, is contraindicated in patients with systemic onset JA after achieving a therapeutic effect. The dose of GC can begin to be slowly reduced only against the background of elimination of systemic manifestations and a clinically significant effect of immunosuppressant therapy for at least one month.
Long-term use of GC, even in low doses, causes the development of serious, often reversible, and in some cases irreversible consequences. The longer patients take GC, the more severe their side effects.
Adverse events:
- short stature. It is not recommended to prescribe GC to children under 5 years of age (especially under 3 years of age), as well as to prepubertal age. The administration of GC can lead to a complete cessation of growth and suppression of the pubertal growth spurt. Children with polyarticular JRA are more likely to develop short stature;
- delayed sexual development;
- arterial hypertension (isolated increase in systolic blood pressure (BP) or increase in systolic and diastolic blood pressure);
- steroid osteoporosis. Develops in all patients treated with prednisolone for a long time. The most rapid loss of bone mass during GC treatment develops during the first 6–12 months from the start of treatment. Therefore, prevention of GC-induced osteoporosis should begin as early as possible. It is most pronounced in the thoracic and lumbar spine. It manifests itself as a decrease in the height of the vertebral bodies, compression fractures. Accompanied by symptoms of compression of the spinal cord roots;
- obesity. Has characteristic features - a moon-shaped face, fat deposition on the neck, chest, abdomen, steroid “hump”, atrophy of the muscles of the arms and legs;
- disproportionate physical development;
- erosive and ulcerative processes in the upper gastrointestinal tract;
- steroid myopathy;
- posterior capsular cataract;
- skin changes (hypertrichosis, purulent skin infection, stretch marks, skin trauma, rough scars, worsening wound healing, steroid acne on the face and torso);
- development of hormone resistance: – continuous relapses of the disease during treatment with maintenance doses of GC;
- development of hormone dependence: – exacerbation of the disease against the background of GC withdrawal;
- withdrawal syndrome.
Intra-articular injection of HA
Local GC therapy quickly relieves acute inflammatory changes in joints and preserves their functional activity. For intra-articular injections, long-acting GCs are used: methylprednisolone, betamethasone, triamcinolone. In patients with oligoarthritis, intra-articular injections of HA prevent disproportionate growth of the lower extremities.
Excessive “passion” for local therapy is unacceptable. GK is administered no more than once every 3–6 months into the same joint. The peculiarities of local GC therapy are that the initial duration of the effect ranges from several weeks to several months. However, in the future, the duration of improvement with repeated administration of drugs without immunosuppressive therapy is reduced, and the patient requires more frequent intra-articular punctures, which leads to the development of traditional adverse effects of GC therapy, including Cushing's syndrome and severe hormone dependence, especially with the introduction of long-acting betamethasone. Doses and indications for use are presented in table. 3 and 4.
Contraindications to local GC therapy:
- local or systemic infection;
- severe bone destruction;
- severe periarticular osteoporosis;
- difficult access to the joint;
- blood clotting pathology;
- ineffectiveness of previous intravenous therapy.
After administration, rest of the joints is required for at least 48–72 hours.
Side effects of intra-articular HA injections:
- “steroid arthropathy” and osteonecrosis;
- iatrogenic infection and hemarthrosis;
- tissue atrophy, lipodystrophy, fat necrosis, calcification;
- tendon ruptures;
- damage to nerve trunks;
- “post-injection” exacerbation;
- erythema, feeling of heat.
In this regard, you can refrain from intra-articular administration of HA. If an adequate dose of an immunosuppressant and/or biological agent is prescribed, the activity of the joint syndrome usually decreases after 2–4 weeks of treatment, and it completely resolves after 6–12 weeks of therapy. If there is pain and stiffness during this period, it is advisable to prescribe NSAIDs, as well as topical ointments and gels containing NSAIDs.
Nonsteroidal anti-inflammatory drugs
The most effective drug with the best tolerability should be selected. When using NSAIDs in rheumatology, one must remember that the development of the anti-inflammatory effect lags behind the analgesic effect. Pain relief occurs within the first hours after administration, while the anti-inflammatory effect develops only after 10–14 days of constant, regular use of NSAIDs.
Treatment should begin with the lowest dose; if well tolerated, the dose can be increased after 2–3 days. In recent years, there has been a tendency to increase single and daily doses of drugs that are well tolerated, while limiting the maximum doses of acetylsalicylic acid, indomethacin, and piroxicam.
For long-term treatment, NSAIDs are taken after meals (in rheumatology). For a quick analgesic and antipyretic effect, NSAIDs are prescribed 30 minutes before meals or 2 hours after meals, washed down with 1/2–1 glass of water. After taking NSAIDs, it is advisable not to lie down for 15 minutes in order to prevent esophagitis. The timing of drug administration may also depend on the time of maximum symptoms, taking into account the chronopharmacology of the drugs. This allows you to achieve the greatest effect with a lower daily dose. For morning stiffness, it is advisable to take rapidly absorbed NSAIDs as early as possible or prescribe long-acting drugs at night.
The most commonly used is Diclofenac sodium at a dose of 2–3 mg/kg body weight per day. In case of severe systemic manifestations, one should refrain from prescribing NSAIDs, as they can provoke the development of macrophage activation syndrome. The dosage regimen for various NSAIDs is presented in table. 5.
The most typical adverse events that occur while taking NSAIDs:
- NSAID gastropathy - indigestion, gastroesophageal reflux, erosion of the upper gastrointestinal tract, gastritis, erosive and ulcerative lesions of the stomach and duodenum, small and large intestines, hemorrhage, bleeding, perforation of stomach and intestinal ulcers;
- liver damage - increased activity of transaminases and other enzymes. In severe cases, jaundice and hepatitis may develop;
- kidney damage: interstitial nephritis - “analgesic nephropathy”. Fluid retention in the body, swelling, increased blood pressure;
- from the central nervous system: headache, dizziness;
- from the hematopoietic system - the development of aplastic anemia and agranulocytosis;
- on the part of the coagulation system - inhibition of platelet aggregation and a moderate anticoagulant effect, bleeding may develop, most often from the gastrointestinal tract;
- hypersensitivity reactions - the appearance of a rash, Quincke's edema, signs of bronchospasm, the development of anaphylactic shock, Lyell's syndrome and Stevens-Johnson.
Immunosuppressive therapy
Immunosuppressive therapy should be differentiated, long-term and continuous, starting immediately after verification of the diagnosis during the first 3-6 months of the disease. The withdrawal of immunosuppressants in most patients causes an exacerbation of the disease.
Methotrexate is a drug from the group of antimetabolites, similar in structure to folic acid, and has a dose-dependent immunosuppressive and anti-inflammatory effect. Methotrexate has a cytotoxic effect in doses above 100 mg/m2/week. In rheumatology, methotrexate is used in doses below 50 mg/m2/week and has a weak immunosuppressive and more pronounced anti-inflammatory effect. Methotrexate reduces disease activity, laboratory activity indicators, and induces seroconversion in the Russian Federation.
Indications:
- youthful (juvenile) rheumatoid arthritis (RF+ and RF-);
- youthful (juvenile) arthritis with systemic onset;
- youthful (juvenile) polyarthritis (seronegative);
- pauciarticular juvenile (juvenile) arthritis.
Treatment regimen:
- Methotrexate is most often prescribed once a week (orally or parenterally). This is due to the fact that more frequent use of the drug is usually associated with the development of acute and chronic toxic reactions. Due to the possible intolerance of simultaneous administration of methotrexate in large doses, it can be prescribed in divided doses, at 12-hour intervals, in the morning and evening or 2 times a week.
- In the majority of patients with the systemic variant of JA, methotrexate in doses of 10–15 mg/m2/week does not significantly affect the activity of systemic manifestations of the disease. For JA with systemic onset, methotrexate is used in doses of 20–25 mg/m2/week, and if ineffective, in the form of pulse therapy in a dose of 50 mg/m2 once a week intravenously for 8 consecutive weeks; when the effect is achieved from the 9th week, methotrexate is administered at a dose of 20–25 mg/m2/week subcutaneously or intramuscularly. For parenteral administration, the contents of the ampoule are dissolved in 400 ml of isotonic sodium chloride solution. The infusion is carried out over 3–4 hours.
- For polyarthritis, methotrexate is used in doses of 15-25 mg/m2/week, for oligoarthritis - 10-15 mg/m2/week.
- The effect is assessed after 4–12 weeks. At these doses, methotrexate does not have a pronounced immunosuppressive effect and stops the destruction of joints in the event of a decrease in laboratory activity indicators. To reduce the side effects of the drug, you should take folic acid 1–5 mg/day on days free from taking methotrexate.
Adverse events:
- headache, blurred vision, drowsiness, aphasia;
- paresis, convulsions;
- interstitial pneumonitis;
- gingivitis, pharyngitis, ulcerative stomatitis;
- anorexia, nausea, vomiting, diarrhea, melena;
- ulceration of the gastrointestinal mucosa, gastrointestinal bleeding;
- liver damage;
- acute renal failure, azotemia, cystitis;
- anemia, leukopenia, thrombocytopenia;
- addition of a secondary (bacterial, viral, fungal, protozoal) infection;
- dysmenorrhea, oligospermia;
- alopecia, ecchymosis, acne, furunculosis.
To relieve adverse events during intravenous administration of methotrexate, it is advisable to premedicate with one of the following drugs:
- Metoclopramide orally, intravenously or intramuscularly. Adults are prescribed 10 mg 3-4 times a day. The maximum single dose is 20 mg, the daily dose is 60 mg. For children from 2 to 14 years of age, a single dose is 0.1 mg/kg body weight, the highest daily dose is 0.5 mg/kg. The frequency of administration is 1–3 times a day.
- Tropisetron orally or intravenously at a dose of 5 mg for adults, for children over 2 years of age - at a daily dose of 0.2 mg/kg, the maximum daily dose is up to 5 mg.
Cyclosporine
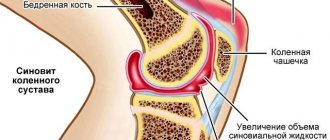
Cyclosporine not only causes symptomatic improvement, but also has a basic antirheumatic effect. Cyclosporine therapy causes a decrease in indicators of disease activity, severity of pain and synovitis, duration of morning stiffness, and improvement in the functional ability of joints. Cyclosporine inhibits the progression of the destructive process in the cartilage and bone tissue of joints and stimulates reparative processes. Cyclosporine improves functional status and minimizes disability in systemic JA. Reduces the rate of increase in structural changes in joints, regardless of the dynamics of laboratory activity indicators. Relieves acute coxitis, stimulates the repair of cartilage and bone in aseptic necrosis of the femoral heads. Cyclosporine is the drug of choice for the treatment of macrophage activation syndrome in systemic JA. Effective for the treatment of uveitis.
Indications:
- youthful (juvenile) arthritis with systemic onset;
- rheumatoid uveitis;
- hemophagocytic syndrome in JA.
Treatment regimen:
- The choice of the initial dose, as well as correction of the dosage regimen during treatment, is carried out taking into account clinical and laboratory parameters.
- The daily oral dose is 3.5–5 mg/kg. The initial dose is 3.5 mg/kg/day. It is divided into two doses (1.5 mg/kg per day every 12 hours). If the number of capsules is not divided by two, then a larger dose is taken in the evening. It should not exceed the morning dose by more than 25 mg.
- For the first 4 weeks, cyclosporine therapy is carried out at a dose of 3.5 mg/kg/day; if there is no effect during the first month of treatment, the dose of the drug is increased by 25 mg. The time period between dose increases should be at least 2 weeks.
- The dose increase is carried out under the control of peripheral blood parameters (number of red blood cells, platelets, leukocytes) and biochemical parameters (concentration of creatinine, urea, bilirubin, potassium, transaminases in the blood serum).
- The daily dose should not exceed 5 mg/kg/day.
- In patients with necrosis of the femoral head or the threat of its development, as well as with the development of hemophagocytic syndrome, the dose of cyclosporine can be increased within the first 2–4 weeks of therapy. Safety indicators in this case should be monitored once every 7–10 days.
- The effect develops after 1–3 months and reaches its maximum within 6–12 months.
Adverse events:
- a feeling of heaviness in the epigastric region, loss of appetite, nausea (especially at the beginning of treatment), vomiting, diarrhea;
- pancreatitis;
- swelling of the gums;
- liver dysfunction;
- headache, paresthesia, convulsions;
- increased blood pressure;
- impaired renal function - so-called nephrotoxicity, leading to an increase in the concentration of creatinine and urea in the blood;
- increased concentrations of potassium and uric acid in the body;
- excessive hair growth;
- reversible dysmenorrhea and amenorrhea;
- slight anemia;
- rarely - muscle spasms, muscle weakness, myopathy, thrombocytopenia.
Cytotoxic agents: cyclophosphamide, chlorambucil, azathioprine are used quite rarely for the treatment of JA due to low efficiency and a high incidence of severe side effects (leukopenia, infections, infertility, neoplastic processes).
Leflunomide
Leflunomide is effective in the treatment of RA in adults. Leflunomide reduces the inflammatory activity of the disease, has a pronounced analgesic effect, reduces the severity of articular syndrome, reduces ESR, circulating immune complexes, RF titers, and stops the progression of osteochondral destruction. The functional ability and quality of life of patients is significantly improved. Leflunomide is effective in both early and late stages of RA. It slows down the progression of joint destruction. The drug is not registered for JRA indications. However, the effectiveness and safety of the drug in children was studied in a double-blind, placebo-controlled study. Given its reliable efficacy and low toxicity, leflunomide can be prescribed if methotrexate is ineffective under the supervision of experienced rheumatologists.
Indications:
- youthful (juvenile) rheumatoid arthritis (RF+ and RF–);
- youthful (juvenile) polyarthritis (seronegative);
- pauciarticular juvenile (juvenile) arthritis, torpid to classical immunosuppressants and biological agents.
Treatment regimen:
- Doses. For body weight above 30 kg: 100 mg once a day for the first 3 days, then 0.6 mg/kg once a day. In children weighing less than 30 kg, the initial dose is 50 mg/day for 3 days, then 0.6 mg/kg/day.
- It is possible to use leflunomide in combination with methotrexate at a dose of 5–7.5 mg/m2/week if leflunomide is insufficiently effective.
Adverse events:
- increased blood pressure;
- diarrhea, nausea, vomiting, anorexia;
- diseases of the oral mucosa (aphthous stomatitis, lip ulcerations);
- abdominal pain;
- liver dysfunction (increased levels of transaminases, alkaline phosphatase, bilirubin);
- slight loss of body weight;
- headache, dizziness, asthenia, paresthesia;
- tenosynovitis;
- increased hair loss, eczema, dry skin;
- leukopenia;
- rash, itching, allergic reactions, urticaria;
- hypokalemia;
- taste disturbance;
- anxiety;
- ligament rupture;
- Stevens-Johnson syndrome;
- toxic epidermal necrolysis, erythema multiforme;
- anemia, thrombocytopenia, pancytopenia, agranulocytosis, eosinophilia.
E. I. Alekseeva, Doctor of Medical Sciences, Professor T. M. Bzarova
NCZD, Moscow
Contact information for authors for correspondence
Use of drugs in the treatment of juvenile arthritis
Drug treatment involves the use of drugs from various groups, each of which helps to accelerate regenerative processes inside the joint and restore its functionality.
IMPORTANT! Self-medication can cause the situation to worsen and trigger irreversible consequences
. Taking medications should be carried out taking into account the recommendations of the attending physician.
Chondroprotectors in the treatment of juvenile arthritis
They enrich the body with the necessary components that enable the regeneration of articular tissues.
The drugs belong to the category of long-acting medications and require many months of use.
The most commonly appointed is considered to be “ Structum”
».
Antispasmodics in the treatment of juvenile arthritis
Excellent helpers in the fight against such an unpleasant symptom as pain.
Taking antispasmodics allows you to get rid of muscle pain caused by pathological processes occurring in the joint tissues.
One of the most effective drugs in this group is “ No-spa
».
Nonsteroidal anti-inflammatory drugs (NSAIDs) in the treatment of juvenile arthritis
The use of NSAIDs has a beneficial effect and helps speed up the recovery process. The action of the drugs is aimed at relieving pain and improving overall well-being.
The duration of use, dosage and frequency of administration are determined by the doctor in accordance with the results of a diagnostic or intermediate examination.
The most effective drug of this type is considered to be “ Artradol”
».
Traditional treatment
Juvenile arthritis is a pathology that requires an integrated approach to the problem. Treatment involves not only suppressing symptomatic signs, but also preventing consequences. Active phase therapy takes place in the hospital, the rest of the time the patient is monitored and sanatorium-resort rest is carried out.
Medication
Depending on the characteristics with which juvenile rheumatoid arthritis develops, therapy is prescribed:
- NSAIDs - anti-inflammatory drugs are taken only after meals. If a quick analgesic effect is needed, then the time of taking the drugs is changed individually, according to the regimen prescribed by the doctor. After use, the child should move for at least 10 minutes to prevent the formation of esophagitis. Treatment is carried out with Piroxicam, Indomethacin, Diclofenac, Ibuprofen, Naproxen. NSAIDs do not affect the progression of the disease, but only relieve the main symptoms.
- Glucocorticosteroids - to suppress the symptoms of the inflammatory process, this pharmacological subgroup is necessary. The active components are quickly eliminated from the body, but the drugs are characterized by a large number of side effects. Long-term therapy with them is prohibited. Treatment is carried out with Prednisolone and Betamethasone.
- Immunosuppressive drugs - drugs are intended to suppress the functionality of the autoimmune system, while protecting joints from destruction. Therapy involves taking it for a long time, and only 3 times a week. The appointment takes into account the individual characteristics of the body, treatment is carried out with Methotrexate, Cyclosporine, Leflunomide.
Important: Therapeutic measures include taking multivitamin complexes in order to stabilize the functioning of internal systems and organs.
Drugs are prescribed according to age and deficiency of certain types of vitamins.
Surgical
Surgery is performed extremely rarely.
Operations are used in exceptional cases when severe joint deformation occurs. Surgical procedures involve partial or complete replacement of the articular apparatus.
Possible complications
Despite the fact that in most patients juvenile arthritis proceeds without complications, with the onset of long-term remission, there is always a risk of complications.
.
Failure to seek medical help in a timely manner, as well as refusal or deviation from prescribed treatment can lead to the development of a number of serious complications, including:
- persistent impairment of joint mobility, causing disability;
- development of cardiopulmonary failure;
- severe impairment of the visual system;
- infectious complications.
Gymnastics
Adolescent juvenile arthritis requires adjustment of habitual physical activity. Patients are prescribed a course of exercise therapy with the need for their daily use, swimming in the pool, exercise on an exercise bike or cycling. The load level has an individual approach program.
Performing gymnastic exercises
Predictions and prevention of juvenile arthritis
More than 50% of officially registered cases can be successfully treated
.
Stable remission can be achieved for a period of up to 10 years. However, despite such positive forecasts, a third of patients become disabled
.
To eliminate the likelihood of irreversible consequences, it is important to exclude factors that could provoke aggravation of the situation. Among them:
- eliminating the possibility of hypothermia, dosing exposure to sunlight;
- maintaining personal hygiene, preventing the likelihood of contact with potential carriers of viruses and infections;
- maximum compliance with safety precautions in order to eliminate the likelihood of injury during sports;
- compliance with the basics of dietary nutrition, enrichment of the diet with calcium and phosphorus.
Clinical guidelines
Due to the fact that the exact causes of the disease cannot be established, clinical recommendations are of an exclusively general nature and are primarily aimed at eliminating the likelihood of a recurrence of exacerbation of the disease.
In order to maintain the achieved treatment results, it is important to combine therapy with observation by a rehabilitation specialist
, which will help develop an action plan to preserve the functionality of the joint.
Among the main recommendations it is worth highlighting:
- increasing vigilance and strengthening measures to ensure sufficient heat levels for the body;
- eliminating the likelihood of stressful situations;
- systematic visits to the attending physician, even with favorable treatment.
Compliance with the recommendations allows you to slow down or completely stop the processes of relapse of pathological processes.
Laboratory diagnostics and instrumental diagnostics
To diagnose the disease, the doctor will prescribe a series of tests.
- General blood analysis. Anemia (decrease in the amount of hemoglobin and red blood cells), leukocytosis (increase in the number of leukocytes), increased erythrocyte sedimentation rate.
- General urine analysis – no changes.
- Blood chemistry.
- Electrocardiogram.
- Sometimes rheumatoid factor can be detected, which indicates a poor prognosis.
- X-ray – narrowing of the joint space, erosion, expansion of the borders of the heart.
Diet for juvenile arthritis
Proper nutrition
– an important stage in the treatment and prevention of existing diseases, in particular pathologies of the musculoskeletal system.
A diet for juvenile arthritis is part of a mandatory therapeutic course, the content of which is determined by the attending physician in order to relieve inflammation of the joints and eliminate unpleasant symptoms.
Among the features of dietary nutrition it is worth highlighting:
- enriching the diet with plant foods in order to replenish the supply of vitamins and minerals;
- limiting the consumption of foods that promote calcium excretion;
- reducing salt intake;
- using gentle cooking methods;
- avoiding alcohol, sugar, carbonated drinks and flour products.
Therapy and procedures
Physiotherapeutic direction involves performing:
- magnetic therapy, electrophoresis - to change the immune status, relieve symptoms of the disease;
- paraffin and mud applications, infrared irradiation - to stabilize motor function, relax muscle tissue;
- cryotherapy, laser therapy - recommended for exacerbations, used to relieve inflammation.
Physiotherapeutic procedures will help relieve pain.
Massage sessions are carried out in the latent phase, they can improve blood circulation in the area of damaged joints, reduce deformation rates, and increase their mobility.