Indications for use
Diseases of the musculoskeletal system (rheumatic lesions of soft tissues, osteoarthritis of peripheral joints and spine, including radicular syndrome, tenosynovitis, bursitis).
Pain syndrome of mild or moderate severity: neuralgia, ossalgia, myalgia, lumboischialgia, post-traumatic pain syndrome (sprains and bruises), accompanied by inflammation, headache, migraine, algodismenorrhea, toothache.
As part of complex therapy for infectious and inflammatory diseases of the ear, nose and throat with severe pain (pharyngitis, tonsillitis, otitis media).
Feverish syndrome with 'colds' and infectious diseases.
Theraliv 275 is used for symptomatic therapy (to reduce pain, inflammation and fever) and does not affect the progression of the underlying disease.
Contraindications
Hypersensitivity to naproxen or naproxen sodium.
Complete or incomplete combination of bronchial asthma, recurrent nasal polyposis or paranasal sinuses and intolerance to acetylsalicylic acid and other non-steroidal anti-inflammatory drugs (NSAIDs) (including a history).
The period after coronary artery bypass surgery.
Erosive and ulcerative changes in the mucous membrane of the stomach or duodenum, active gastrointestinal bleeding.
Inflammatory bowel diseases (ulcerative colitis, Crohn's disease) in the acute phase.
Hemophilia and other bleeding disorders and hemostasis disorders.
Cerebrovascular bleeding or other bleeding.
Decompensated heart failure.
Severe liver failure or active liver disease.
Severe renal failure (creatinine clearance (CC) less than 30 ml/min), progressive kidney disease, confirmed hyperkalemia.
Pregnancy, breastfeeding period.
Children under 15 years of age.
Carefully
Coronary heart disease, cerebrovascular diseases, congestive heart failure, dyslipidemia/hyperlipidemia, diabetes mellitus, peripheral arterial disease, smoking, renal dysfunction (creatinine clearance 30-60 ml/min), anamnestic data on the development of ulcerative lesions of the gastrointestinal tract (GIT) , presence of Helicobacter pylori infection, use in elderly patients, systemic lupus erythematosus or mixed connective tissue diseases (Sharpe's syndrome), long-term use of NSAIDs, frequent alcohol consumption, severe somatic diseases, concomitant therapy with the following drugs: anticoagulants (for example, warfarin), antiplatelet agents (eg, acetylsalicylic acid, clopidogrel), oral glucocorticoids (eg, prednisolone), selective serotonin reuptake inhibitors (eg, citalopram, fluoxetine, paroxetine, sertraline).
Forsteo®
| Mechanism of action |
Teriparatide is a recombinant human parathyroid hormone produced using a strain of Escherichia coli (using DNA recombination technology).
Endogenous parathyroid hormone (PTH), which is a sequence of 84 amino acid residues, is the main regulator of calcium and phosphorus metabolism in the bones and kidneys. Teriparatide (recombinant human PTH (1-34)) is an active fragment of endogenous human PTH. The physiological effect of PTH is to stimulate bone formation through a direct effect on osteoblasts. PTH indirectly increases intestinal absorption and tubular reabsorption of calcium, as well as renal excretion of phosphate. Pharmacodynamic properties The biological effect of PTH is due to binding to specific PTH receptors on the surface of cells. Teriparatide binds to the same receptors and has the same effects on bones and kidneys as PTH.
Single daily administration of teriparatide stimulates the formation of new bone tissue on the trabecular and cortical (periosteal and/or endosteal) surfaces of bones with preferential stimulation of osteoblast activity relative to osteoclast activity. This is confirmed by an increase in the levels of markers of bone tissue formation in the blood serum: bone-specific alkaline phosphatase and carboxy-terminal propeptide of type I procollagen (PICP). The increase in the content of bone tissue formation markers is accompanied by a secondary increase in the level of bone resorption markers in urine: N-telopeptide (NTX) and deoxypyridinoline (DPD), which reflects the physiological interaction of the processes of bone tissue formation and resorption in skeletal remodeling. 2 hours after administration of teriparatide, a short-term increase in serum calcium concentration is observed, which reaches maximum values after 4-6 hours and returns to baseline values within 16-24 hours. In addition, transient phosphaturia and a slight short-term decrease in serum phosphorus levels may occur.
Clinical effectiveness
Postmenopausal osteoporosis
The main clinical trial of teriparatide included 1637 patients with postmenopausal osteoporosis, with a mean age of 69.5 years.
At the start of the study, 90% of patients had suffered 1 or more vertebral fractures and the mean vertebral bone mineral density (BMD) was equivalent to a T-score of -2.6. All patients took 1000 mg of calcium daily and. at least 400 IU vitamin D.
Results of teriparatide therapy for up to 24 months (mean duration of therapy was 19 months) indicate a statistically significant reduction in the incidence of fractures. The incidence of new vertebral fractures (>1 fracture, as measured by radiography at the beginning and end of the study) in the teriparatide group and in the placebo group was 5.0% and 14.3%, respectively (p < 0.001 compared with the placebo group, a relative decrease risk - 65%).
The incidence of multiple vertebral fractures (>2 fractures, as determined by radiography at the beginning and end of the study) in the teriparatide group and in the placebo group was 1.1% and 4.9%, respectively (p < 0.001 compared with the placebo group, relative risk reduction - 77%).
The incidence of nonvertebral low-energy fractures (minimal trauma fractures) in the teriparatide group and placebo group was 2.6% and 5.5%, respectively (p < 0.025 compared with placebo group, relative risk reduction 53%).
The incidence of major nonvertebral low-energy fractures (femur, radius, humerus, ribs, pelvis) in the teriparatide group and placebo group was 1.5% and 3.9%, respectively (p < 0.025 compared with the placebo group, decreased relative risk - 62%).
After 19 months of treatment (median duration of therapy), there was an increase in BMD at the lumbar spine and proximal femur compared to placebo by 9% and 4%, respectively (p < 0.001). Post-therapy follow-up: After completion of teriparatide therapy, 1262 women with postmenopausal osteoporosis from the main study were included in a follow-up study. The primary objective of the study was to collect data on the safety of teriparatide. During this observation period, other osteoporosis therapy was permitted and additional evaluation of vertebral fractures was performed. At an average of 18 months after discontinuation of teriparatide therapy, the number of patients with at least one new vertebral fracture in the teriparatide-experienced group was 41% lower than in the placebo group (p=0.004).
| In an open-label study, 503 patients with postmenopausal severe osteoporosis and fragility fractures (minimal trauma fractures) within the previous three years (83% had previously received treatment for osteoporosis) received teriparatide for 24 months. After 24 months, BMD at the lumbar spine, proximal femur, and femoral neck increased from baseline by an average of 10.5%, 2.6%, and 3.9%, respectively. From 18 to 24 months, BMD at the lumbar spine, proximal femur, and femoral neck increased by 1.4%, 1.2%, and 1.6%, respectively. Osteoporosis in men |
In a clinical study of men with osteoporosis due to hypogonadism (defined by low morning free testosterone or elevated follicle-stimulating hormone or luteinizing hormone concentrations) or
| 437 patients with idiopathic osteoporosis took part, with an average age of 58.7 years. At the start of the study, the BMD of the vertebrae and femoral neck according to the T-criterion ranged from -2.2 to -2.1, respectively. At the start of the study, 35% of patients had a history of vertebral fractures, and 59% of patients had fractures of other locations. All patients took 1000 mg calcium and at least 400 IU vitamin D daily. Significant increases in lumbar spine bone mineral density were noted after 3 months. After 12 months of therapy, BMD at the lumbar spine and proximal femur increased by 5% and 1%, respectively, compared with placebo. |
Osteoporosis with long-term therapy
| glucocorticosteroids The effectiveness of teriparatide in osteoporosis caused by long-term treatment with glucocorticosteroids was proven in an 18-month randomized, double-blind clinical trial with an active comparator (alendronate 10 mg/day; 428 patients, mean age 57 years). At the start of the study, 28% of patients had 1 or more vertebral fractures. All patients took 1000 mg of calcium and 800 IU of vitamin D daily. The study included 277 postmenopausal women, 67 premenopausal women and 83 men. After 18 months of therapy, BMD of the lumbar spine increased by 7.2% (by 3.4% in the alendronate group, p < 0.001), BMD of the proximal femur increased by 3.6% (by 2.2% in the alendronate group, p<0.01), femoral neck BMD increased by 3.7% (by 2.1% in the alendronate group, p<0.05). |
In patients taking teriparatide,
| study period from 18 months to 24 months of therapy, BMD of the lumbar spine, proximal femur, and femoral neck increased by an additional 1.7%, 0.9%, and 0.4%, respectively. In the teriparatide group, after 36 months of therapy, new vertebral fractures were detected in 1.7% of patients (7.7% in the alendronate group, p = 0.01), new non-vertebral fractures were detected in 7.5% of patients (7.0% in the alendronate group, p =0.84). In premenopausal women after 18 months of therapy, the increase in BMD was significantly higher in the teriparatide group compared with alendronate: lumbar spine BMD increased by 4.2% (-1.9% in the alendronate group, p < 0.001), proximal BMD femur increased by 3.8% (0.9% in the alendronate group, p = 0.005). |
Mineralization processes occur without signs of toxic effects on bone tissue cells, and bone tissue formed under the influence of teriparatide has a normal structure (without the formation of reticulofibrous bone tissue and bone marrow fibrosis). Teriparatide reduces the risk of fractures regardless of age, baseline bone turnover, or BMD (relative risk reduction for new fractures is 65%).
How to use: dosage and course of treatment
The drug is taken orally. The tablets should be taken with enough water.
Adults and children 15 years and older
Typically, the daily dose used to relieve pain is 2-3 tablets (550-825 mg). The maximum daily dose is 3 tablets (825 mg). Duration of use - no more than 5 days.
When using Theraliv 275 as an antipyretic, the initial dose is 2 tablets, then 1 tablet (275 mg) is taken every 8 hours.
For the prevention and treatment of migraine attacks, the initial recommended dose is 2 tablets (550 mg), if necessary, you can take 1 tablet (275 mg) every 8-12 hours. The maximum daily dose is 3 tablets (825 mg).
To relieve menstrual pain and cramps, pain after insertion of intrauterine devices and other gynecological pain, it is recommended to prescribe the drug in an initial dose of 2 tablets (550 mg), then 1 tablet (275 mg) every 8 hours.
Children
Theraliv 275 is contraindicated for use in children under 15 years of age.
Elderly patients (≥65 years)
Patients over 65 years of age should take the drug as needed every 12 hours.
To reduce the risk of developing adverse events from the gastrointestinal tract, the drug should be taken in the minimum effective dose for the shortest possible short course.
If you feel that the effect of the drug is very strong or weak, tell your doctor or pharmacist.
Overdose
Symptoms
A significant overdose of naproxen may be characterized by drowsiness, dyspeptic disorders (heartburn, nausea, vomiting, abdominal pain), weakness, tinnitus, irritability, and in severe cases, hematemesis, melena, impaired consciousness, convulsions and renal failure.
Treatment
A patient who has accidentally or intentionally taken a large amount of Theraliv 275 needs to lavage the stomach, take activated charcoal and undergo symptomatic therapy: antacids, H2 receptor blockers, proton pump inhibitors. Hemodialysis is ineffective.
Forsteo
The parathyroid hormone analogue, which is a sequence of 84 amino acid residues, is the main regulator of Ca2+ and phosphorus metabolism in bone tissue and kidneys. The active substance of the drug is an active fragment of endogenous human parathyroid hormone. The physiological effect of parathyroid hormone is to stimulate bone formation through a direct effect on osteoblasts. Indirectly increases intestinal absorption and tubular reabsorption of Ca2+, as well as phosphate excretion by the kidneys.
The biological effect of parathyroid hormone is carried out by binding to specific receptors on the surface of cells. The active substance of the drug binds to the same receptors and has the same effect on bone tissue and kidneys as parathyroid hormone. Daily single administration of the drug stimulates the formation of new bone tissue on the trabecular and cortical (periosteal and/or endosteal) surfaces of bones with preferential stimulation of osteoblast activity relative to osteoclast activity. This is confirmed by an increase in the content of markers of bone tissue formation in the blood serum: bone-specific ALP and procollagen-1 carboxy-terminal propeptide. An increase in the content of bone tissue formation markers is accompanied by a secondary increase in the concentration of bone resorption markers in urine: N-telopeptide and deoxypyridinoline, which reflects the physiological interaction of the processes of bone tissue formation and resorption in skeletal remodeling. 2 hours after administration of the drug, a short-term increase in the concentration of serum Ca2+ is observed, which reaches maximum values after 4-6 hours and returns to the initial level within 16-24 hours. In addition, transient phosphaturia and a slight short-term decrease in the concentration of phosphorus in the blood serum may be observed. .
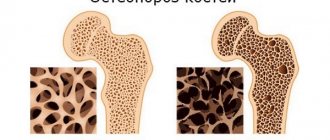
During treatment with the drug, bone mineral density (BMD) of the whole body increases by 5-10% (including the lumbar spine, femoral neck and femur).
Mineralization processes occur without signs of toxic effects on bone tissue cells, and the bone tissue formed under the influence of the drug has a normal structure (without the formation of reticulofibrous bone tissue and bone marrow fibrosis).
The use of the drug reduces the risk of developing fractures regardless of age, the initial level of bone metabolism or BMD (the relative reduction in the risk of new fractures is 65%).