acupuncture 2100 rub.
massage 1800 rub.
113 reviews
If you are in your twenties and a woman, your chances of experiencing knee pain are 1:5. Perhaps an orthopedist or surgeon told you that you have “deforming gonarthrosis of the knee joint”, recommended treatment, assigned the disease grade 1, 2 or 3, named possible causes, and confirmed the symptoms. And most likely, you already know what it’s like when pain keeps you awake at night, makes it difficult to move, and even just to live. Let's figure out what gonarthrosis is, why you are unlucky to get it, and how you can reverse the process without drugs and harm to your health.
Analyzing a person as an integral system, experts consider gonarthrosis of the knee joint to be a suffering of the whole organism. The most common cause of gonarthrosis is a hormonal imbalance in the functioning of internal organs. As a rule, it all starts with the kidneys. The kidneys are the body's filter. Their task is to filter the blood and free the blood from salts. If the kidneys fail to cope with their function, the blood is not cleansed and salts remain. To protect yourself from blood and salt entering the brain, the body “stores” salts in the joints (gonarthrosis) and the spine (osteochondrosis).
When the process has gone far, arthritis occurs. Then the blood vessels narrow, also to keep salt out. All this leads to poor blood supply to the muscles, increasing the load on the skeleton - the spine, joints, intervertebral discs. Then the liver, gallbladder, and pancreas are added. Joints lose flexibility, muscles atrophy. Then the body “wears out” and the disease progresses.
For the treatment of gonarthrosis at the East Clinic we use:
Most likely, they have already tried to treat gonarthrosis. They took medications, went to physical therapy, had massages. Received a temporary effect or did not receive it at all. And you got the side effects to the fullest, right? And now a joint replacement operation is looming on the horizon. Of course, in the last stage there is no other way out. It is in your power to prevent such a situation and begin the fight against gonarthrosis according to the rules. At East Clinic we use complex treatment, which produces results from the first sessions for most patients.
Therapeutic massage, osteopathy, soft manual techniques.
First, the muscles are warmed up with a massage. Usually they work on biologically active points. The doctor finds painful areas and performs certain movements, deeply working the muscles, restoring the correct position of the joint. Thanks to this work, it is possible to restore range of motion, relieve pain, and eliminate deformities. Of course, the first sessions are quite painful. However, as you recover, the pain disappears, and the ability to live fully without pain returns.
Acupuncture for a “bouquet” of diseases.
Needles are inserted into special points depending on the condition. Each of more than 400 biologically active points is associated with an organ, so before the procedure, diagnostics are carried out to determine which points are needed to work. With the help of acupuncture, you can relieve inflammation, improve tissue nutrition and metabolism, and restore immunity. And also - treat concomitant diseases, improve sleep and mood.
Hirudotherapy.
Leeches eliminate muscle spasms, restore lymph and blood circulation in the area of the diseased joint, and stimulate the restoration of damaged cartilage tissue.
Warming up for pain and spasms - moxotherapy.
Warming certain points with a wormwood cigar - moxa - gives quick results. The effect, as with acupuncture, appears not only at the site of impact. Warming with moxa speeds up blood flow and metabolism. Gives you the opportunity to move more freely, feel more energetic and active.
Laser on points - laserotheropuncture.
This is a combination of low intensity laser effect and acupuncture effect. This combination helps to begin the restoration of damaged cartilage, remove swelling, pain and inflammation. And all this is comfortable, without pain. Therefore, laser is possible for those who are not suitable for other methods.
Homeopathic injections for gonarthrosis - homeosynia.
Traditionally, injectable drugs from Heel are used for treatment with this method. These are complex preparations of herbs, minerals, substances of animal origin and much more. Some drugs contain dozens of components. The doctor selects a regimen and combination of medications. For deforming gonarthrosis, homeosynia is one of the most effective methods.
And, of course, every patient with gonarthrosis receives nutritional recommendations and a set of physical exercises. For joints, movement is life in the truest sense.
Treatment of gonarthrosis of the knee joint 2 degrees
Drug treatment
This type of treatment consists of prescribing and using various medications under the constant supervision of a specialist. It depends on your doctor which medications will be most successful in performing their functions.
Anti-inflammatory drugs
These medications are designed to relieve pain. They are more likely to reduce symptoms and have a beneficial therapeutic effect.
Treatment with chondroprotectors
The use of chondroprotectors helps restore cartilage. Chondroprotectors are natural, easily accepted by the body and stimulate collagen synthesis well. However, you need to know that these drugs are recommended to be taken regularly. Treatment lasts quite a long time. Chondroprotectors also include preparations containing hyaluronic acid.
All of these medications act slowly and are mainly available in the form of injections. It is this method of using the drug that in this case has the best effect.
Painkillers
Usually, so-called intra-articular injections are prescribed for this purpose. Corticosteroids are used for this. These should be taken in short courses and used only for pronounced symptoms and fairly severe pain.
Physiotherapy for gonarthrosis
Several types of ointments are used to complete this course. They are more of a warming drug than anti-inflammatory in nature. The purpose of these remedies is to increase blood circulation and eliminate inflammation.
Orthopedic treatment
Orthopedic treatment involves the appointment by an orthopedic surgeon and the use of certain shoe insoles, knee pads, and canes, which remove excess stress from sore knees, making walking easier.
Massage
One very important complementary treatment method is massage. The course of treatment is prescribed by the doctor. It usually consists of 5-10 sessions and is prescribed in the complete absence of inflammation. Lymphatic drainage massage also works effectively. It prevents fluid from accumulating in the knee joint. Manual therapy combines rubbing and stroking. This procedure has the greatest effect after a physical therapy session.
Injections
Injections in the treatment of grade 2 knee joint gonarthrosis help to quickly and effectively deliver the drug to damaged tissues. They are indicated for acute joint pain, but in themselves are not the basis of therapy. Injections can only be considered as a very successful addition to the general course of treatment. However, they are the ones that help relieve pain the fastest. In addition, injections for the knee joints can reduce the dose of oral medications. And this is very important if side effects occur with disruption of the gastrointestinal tract.
Treatment with folk remedies
Traditional medicine also knows how to treat grade 2 gonarthrosis of the knee joint. The main tool here is clay.
Recipe: wipe the joint with a damp cloth, dilute the clay with water to the consistency of sour cream. Spread the clay on a cotton cloth in a 2cm layer. Apply this compress to the sore knee and bandage it. Insulate the joint with a wool scarf. Leave the compress on for 2 hours. Afterwards, wash your knee with warm water. Most likely, you will need to do about 5 of these procedures daily.
Exercises for grade 2 knee gonarthrosis
Before treating grade 2 gonarthrosis of the knee joint with exercises, you must consult a doctor. Gymnastics requires a special approach. The whole process should be easy and enjoyable, not painful. If you experience the slightest pain, you must stop exercising immediately.
A set of exercises is prescribed individually. This depends on the patient's condition and physical fitness.
Why is complex treatment at East Clinic more profitable and effective?
To make gonarthrosis a thing of the past as quickly as possible, we offer a range of treatment procedures. This saves you time, money and speeds up your recovery. Treatment of gonarthrosis works faster when combining massage, osteopathy, gentle manual therapy, acupuncture with laser, moxotherapy, and homeosinology. These procedures are included in the course of treatment sessions, the combination of which is selected by the doctor.
During one visit to the clinic, you can undergo two or more procedures, and if you pay for a course of treatment at a time, you will receive a pleasant bonus - a 10% discount. The cost of one treatment session depends on the set of procedures.
The rate of cure using non-medicinal methods depends on the person’s life potential, but in most cases, in stage 2, one comprehensive course of treatment is sufficient.
Gonarthrosis of the knee joint: causes and mechanisms. Why you?
There are a lot of reasons. These include excess weight, problems with the endocrine system, injuries, and previous inflammation of the joint - arthritis. To understand why gonarthrosis appears, let's look at how the joint works in health and disease.
The main load in the joint during movement falls on the articular cartilage. It is like a sponge soaked in joint fluid: it contracts during exercise - the fluid is squeezed out, it expands during rest - the fluid is absorbed back. There are no vessels in the cartilage; all nutrition comes from the fluid inside the joint. If the load is greater than the ability to recover, and nutrition is insufficient, the cartilage suffers. Microscopic cracks appear on it, it becomes more fragile and thinner. This is the beginning of the path to gonarthrosis.
Drug treatment of osteoarthritis
ABOUT
Steoarthrosis (OA) is a heterogeneous disease that occurs most often in people over 60 years of age and is characterized by severe pain and often the presence of inflammatory signs that bring the patient to the doctor.
Pain is the most common symptom in OA
, so treatment of the disease is aimed primarily at reducing pain and, as a result, improving joint function.
10–30% of patients with OA develop varying degrees of disability, so the ability to influence the natural history of the disease would be of great social and economic importance. Traditional treatment for OA
consists primarily of pain management, which begins with various
non-pharmacological methods
, including patient education, physical therapy, transcutaneous stimulation, and others.
However, if non-pharmacological treatment methods are insufficiently effective, drug therapy is required. The action of most drugs is aimed primarily at treating the symptoms of the disease, although some of them are considered as drugs that affect the catabolic and anabolic processes that occur when cartilage is damaged. These drugs are classified as disease-modifying drugs. The choice of drugs and the selection of combinations of various treatment methods remain strictly individual (Table 1). Knowledge of the mechanisms of action, effectiveness, contraindications when prescribing drugs, and the safety profile of drugs is extremely important. Despite the relatively long period of drug use, many questions remain regarding symptomatic treatment. Hierarchically, the first place is occupied by analgesic drugs
. The use of acetaminophen in OA was first recommended by Bradley et al. [1], who showed that 4.0 g of acetaminophen per day was comparable in effectiveness to two doses of ibuprofen (1200 mg and 2400 mg) in patients with OA of the knee and hip joints, and the drug was better tolerated. A study of the mechanism of action of the drug revealed the presence of high analgesic, antipyretic and slight anti-inflammatory activity [3,4]. In vitro, high doses of acetaminophen inhibited prostaglandin synthesis [5]. Acetaminophen appears to act on the central nervous system through inhibition of prostaglandin E2 synthesis and has no effect on prostaglandin synthesis in peripheral tissues [b].
Further study of acetaminophen in terms of its effectiveness, the risk of adverse reactions, as well as the cost of treatment, showed the advantage of its use in patients with OA with mild to moderate pain, but it was noted that clinical effectiveness is higher with non-steroidal anti-inflammatory drugs
(NSAIDs) [2]. In addition, there is evidence of hepatotoxicity and the potential damaging effects of acetaminophen on the kidneys [7], in connection with which attention is drawn to the fact that when taking about 60 g of alcohol per day, the dose of acetaminophen should not exceed 2.0 g per day. The advantage of the drug over other analgesics is its low toxicity for the gastrointestinal tract (GIT), however, in patients with severe pain and inflammation, analgesics are not effective enough and the prescription of NSAIDs is required.
NSAIDs, having anti-inflammatory, analgesic and antipyretic properties, are widely used to reduce pain, gel syndrome, and improve joint function in patients with OA. The choice of NSAID is usually based on the opinion of the doctor. At therapeutic doses, the clinical effectiveness of NSAIDs is approximately the same, but individual response to drugs varies widely [8]. Differences in clinical effectiveness are also influenced by the pharmacokinetic, pharmacodynamic and metabolic properties of the drugs. Although the variability in response can be explained (in part) by drug adsorption, distribution and metabolism, the most important reason for the differences in effectiveness appears to lie in potential differences in the mechanism of action of the drugs [9].
Most NSAIDs are weak organic acids and are completely absorbed when taken orally, about 95% of the drug taken is bound to serum albumin, so hypoalbuminemia, especially in older people with chronic somatic diseases, can lead to increased serum concentrations of NSAIDs and, as a consequence, to increased toxicity. Taking NSAIDs with food can reduce its absorption, as for example, this is shown for naproxen, the adsorption of which is reduced by 16% under these conditions. NSAIDs are metabolized mainly in the liver and excreted in the urine, which must be taken into account when prescribing these drugs to patients with impaired liver or kidney function. Plasma concentrations of drugs vary widely and depend on renal clearance and metabolic rate.
The residence time of drugs in plasma is of some importance in explaining the various clinical effects, according to which NSAIDs are divided into “short- and long-lived”.
The anti-inflammatory and analgesic activity of NSAIDs is associated with a decrease in the production of prostaglandins E. NSAIDs have also been shown to inhibit the formation of prostacyclin and thromboxane, exerting a complex effect on vascular permeability and platelet aggregation. The anti-inflammatory effect of NSAIDs is apparently due to inhibition of cyclooxygenase activity
(COX) is the main enzyme in the metabolism of arachidonic acid on the path of its conversion into prostaglandins.
There are at least two isoforms of COX. And although their amino acid sequence is 60% identical, they are the product of two different genes. COX-1
regulates normal cellular processes and is stimulated by hormones or growth factors. COX-1 is expressed on most tissues of the body and is inhibited to varying degrees by all NSAIDs, so many adverse reactions from the gastrointestinal tract are explained by this inhibition [13].
Another isoform, COX-2
, which is usually not detected in tissues, is expressed during inflammation and is also inhibited by all existing NSAIDs to a greater or lesser extent, as well as by glucocorticosteroids [34], which distinguishes it from COX-1.
Based on these data, a new group of drugs was created - specific COX-2 inhibitors, which include celecoxib and rofecoxib, which are approximately 300 times more effective in suppressing COX-2 compared to COX-1 [15]. Clinical studies in OA showed that both drugs had an effect on pain comparable to the effect of naproxen, ibuprofen and diclofenac, and had almost no damaging effect on the gastrointestinal mucosa and platelet aggregation [16,17,18].
However, evidence is accumulating that the anti-inflammatory and analgesic effects of NSAIDs are not explained solely by COX inhibition. It is assumed that NSAIDs inhibit the activation and chemotaxis of neutrophils and reduce the production of toxic oxygen radicals in stimulated neutrophils [5], inhibit the activity of the transcription factor NF-kB, thus inhibiting the stimulation of nitric oxide synthetase [20].
In addition, NSAIDs, being inhibitors of prostaglandins, which are believed to inhibit apoptosis (programmed cell death), can help normalize the life cycle of cells at the site of inflammation [14].
Arachidonic acid is exposed not only to COX, but also to 5- and 12-lipoxygenases. To date, evidence is accumulating that the mechanism of development of NSAID-induced gastropathy may include alternative processes of conversion of arachidonic acid into leukotrienes under the influence of 5-lipoxygenase (5-LOX). This was the reason for the creation of a new group of drugs capable of inhibiting COX-1 and COX-2. The first study of such a drug - licofelone
(200 mg x 2 times a day) was carried out in 148 patients with OA in a comparative trial with naproxen (500 mg x 2 times a day) and placebo [19]. Licofelon turned out to be equal in effectiveness to NSAIDs, was extremely well tolerated and did not cause damage to the gastrointestinal tract,
Thus, the study of the mechanisms of action of NSAIDs led to the creation of a new classification of drugs (Table 2), based on the selectivity of action against COX, allowing the doctor to individually select the most effective and safe drug.
Numerous controlled studies indicate a significantly higher effectiveness of NSAIDs compared to placebo, therefore, when choosing a drug, it is necessary to take into account its safety, effect on cartilage, and, given the age of patients with OA, and the presence of a large number of concomitant pathologies, compatibility with other drugs.
The general principles of using NSAIDs for OA are to use the minimum effective dose, take no more than one NSAID at a time, discontinue the drug if there is no pain, and evaluate the effectiveness of treatment after 2-4 weeks from the start of use.
For OA, the most optimal are “short-lived” drugs: ibuprofen, diclofenac, ketoprofen. The doses of drugs used for OA are usually lower than for inflammatory diseases of the joints (Table 3). To quickly relieve pain, drugs with high analgesic activity are prescribed: potassium salt of diclofenac (Rapten Rapid)
. The drug has a persistent analgesic effect much faster and longer than the sodium form of diclofenac and other NSAIDs. In addition, the drug has minimal side effects compared to ibuprofen and indomethacin. Rapten Rapid can also be recommended for introductory therapy to relieve pain with subsequent transition to another analgesic. A decrease in pain during treatment with NSAIDs is observed in approximately 60–70% of patients with OA. At the same time, individual differences in the effectiveness of NSAIDs vary widely, so the first place comes not only to the selection of the most effective drug (Rapten Rapid, etc.), but also to the assessment of real differences in the toxicity of drugs. Adverse reactions observed with NSAID treatment are presented in Table 4.
The most clinically significant are adverse reactions from the gastrointestinal tract; their occurrence is likely due to local and systemic inhibition of prostaglandin synthesis.
The spectrum of damage to the gastrointestinal tract is quite wide - from the development of esophagitis to bleeding and perforation of peptic ulcers, sometimes leading to death [13]. Data are provided on damage to the mucosa of the small and large intestines [23]. Endoscopic studies have shown that the prepyloric and antral parts of the stomach are most often affected. They are usually asymptomatic, making it difficult to estimate their true prevalence. Risk factors for the development of NSAID gastropathy include the age of patients over 60 years of age, a history of ulcers, taking antiulcer drugs for any reason, the combined use of glucocorticosteroids, and the presence of severe concomitant diseases, for example, cardiovascular [21,22]. Based on numerous clinical studies, the relative risk for gastric ulcers is 4.0–5.0; for duodenal ulcers – 1.1–1.6 and from 4.5 to 5.0 – for gastric ulcers with bleeding, perforation or death. “Long-living” NSAIDs, such as piroxicam, have greater gastrointestinal toxicity. Significantly better results were obtained using predominantly selective COX-2 inhibitors: meloxicam and nimesulide. Individual clinical studies indicate similar efficacy to standard NSAIDs, but higher safety in relation to gastrointestinal lesions.
The use of specific COX-2 inhibitors is associated with a significant reduction in the incidence of gastrointestinal ulcers compared with diclofenac and ibuprofen. In addition, severe gastroenterological adverse reactions (bleeding, perforation) occur 2–3 times less often when taking rofecoxib and celecoxib. In recent years, questions about the risk/benefit of NSAIDs in OA and the costs of gastroenterological adverse reactions have been constantly discussed. Apparently, selective COX-2 inhibitors can significantly improve the safety of treatment, especially in elderly patients with high risk factors.
If NSAIDs are insufficiently effective and poorly tolerated, other analgesic drugs are prescribed. Tramadol
– a synthetic analgesic of central action, which does not cause physical and mental dependence, is effective for pain of various origins. Schnitzer et al. [24] showed that the drug is effective against pain; in addition, the combined use of tramadol with naproxen made it possible to reduce the dose of the latter. Tramadol is used for OA in doses of 50 to 200 mg per day. Relatively common adverse reactions when using the drug (nausea, dizziness, constipation and drowsiness) can be significantly reduced by titrating the dose (over 3 days, increasing the dose by 25 mg).
In case of isolated articular damage, local remedies are used for the treatment of OA in the form of ointments, NSAID-based creams, which avoids systemic adverse reactions, especially in the elderly, and chondroitin sulfate in combination with dimethyl sulfoxide ( Chondroxide
). A form of NSAID in the form of a patch containing diclofenac has been created, which turned out to be more effective than placebo in a 2-week study in 155 patients with gonarthrosis [25], which may reduce the need for systemic drugs.
Moore et al. analyzed 86 studies to assess the effectiveness and toxicity of topical agents for the treatment of acute and chronic pain, including OA. The authors concluded that these drugs are quite effective and safe [26].
In patients with gonarthrosis in the presence of joint effusion, intra-articular injections of corticosteroids
. Unfortunately, certain aspects of this therapy remain controversial (its relative effectiveness, potentially damaging effects and potentially structure-modifying effects), so the place of long-term use of intra-articular corticosteroid therapy for the treatment of OA remains unclear - long-term double-blind studies are required. Currently, it is believed that the number of intra-articular injections into one joint should not exceed 3-4 over the course of one year.
Although NSAIDs reduce pain and inflammation, it has not yet been shown whether they slow the progression of OA or protect cartilage from mechanical or inflammatory damage.
Attempts to influence the disease began with the study of risk factors, such as obesity, excess joint stress, and were limited to recommendations for weight loss and the use of various physical exercises. Currently, agents are being developed that can slow the progression of OA. The range of drugs being studied is quite wide: from antibiotics with anti-collagenolytic activity, hyaluronate and polysaccharides to more complex drugs, including growth factors and cytokines. These drugs are called structure-modifying drugs, their role is the ability to prevent, slow down, stabilize or reverse the development of the osteoarthritis process not only in cartilage, but also in the entire joint, considered as an organ as a whole. Currently, there is no clear evidence of structure-modifying properties for humans for any drug, but certain data have been obtained for some of them.
Chondroitin sulfate
(CS) and
glucosamine sulfate
(GS) are sulfated glycosaminoglycans that are found in the extracellular matrix of articular cartilage. When taken orally, they are well adsorbed and found in high concentrations in the joint cavity. The mechanism of their action is not fully understood. Evidence has been obtained that cholesterol and HS stimulate the synthesis of hyaluronic acid and proteoglycans and inhibit the action of proteolytic enzymes, thereby enhancing anabolic processes in cartilage and suppressing catabolic ones, which may underlie their structure-modifying effect. There is evidence of their ability to suppress the formation of superoxide radicals and the synthesis of nitric oxide, which apparently determines their analgesic effect. In vivo experimental studies found that oral or intramuscular administration of cholesterol to rabbits (with cartilage degeneration caused by chymopapain) significantly increased the content of cartilage proteoglycans compared to control animals, indicating that cholesterol protects cartilage when damaged and has the ability to support resynthesis of matrix proteoglycans [27].
Treatment of cholesterol leads to a significant reduction in pain, improvement in the functional ability of joints and allows a reduction in the dose of NSAIDs taken. The drug is well tolerated, causing virtually no side effects [28,29,30]. A meta-analysis of double-blind, placebo-controlled studies of cholesterol in 372 and 404 patients with gonorrhea and coxarthrosis confirmed the effectiveness of the drug compared to placebo and NSAIDs [31,32]. A study conducted in Switzerland [33] over the course of a year raised the question of whether cholesterol has structure-modifying properties, since the drug had a stabilizing effect on the width of the joint space and metabolic processes in the subchondral bone and cartilage, and thus, apparently, on the course of the disease. At the annual European Congress of Rheumatology, held in Stockholm (2002), the results of long-term, for 2 years, use of cholesterol (800 mg/day) in 300 patients with gonarthrosis were reported, which showed the ability of the drug to slow down the progression of OA [34 ].
Earlier short-term studies showed the effectiveness of GS compared with placebo and NSAIDs [35,36]. Recently, long-term (for 3 years) use of GS (1500 mg/day) in 212 patients with gonarthrosis confirmed not only the symptomatic effect of the drug (decrease in the functional WOMAC index), but also its ability to slow down radiological progression, which was assessed by changes in the width of the joint gaps [37].
Relatively recently, work on the combined use of
these drugs.
Thus, a double-blind placebo-controlled study for 16 weeks of a combination of glucosamine hydrochloride
(1500 mg/day) and
cholesterol
(1200 mg/day) in 34 men with gonarthrosis and pain in the lower back showed a significant reduction in joint pain, but not in back [38]. Another study reported combination therapy for temporomandibular joint OA [39]. However, promising data on the structure-modifying effects of cholesterol and GS require further confirmation in long-term controlled studies.
Hyaluronate
(GL) is a polysaccharide consisting of a long chain of disaccharides, produced in joints by chondrocytes and synovial cells. Currently, 2 GL drugs are used, administered intra-articularly: low molecular weight (m.v. 500,000–730,000 daltons) and high-molecular weight (m.w. 6,000,000 daltons). The clinical use of GL was due to studies of synovial fluid in OA, which showed a significant change in its rheological properties compared to synovial fluid of a healthy joint. Therefore, it was assumed that the introduction of GL would restore the protective and so-called shock-absorbing properties of synovial fluid [40]. However, the long-term clinical effect could not be explained by these reasons alone, since the half-life of the drug was 2–8 days. Based on further studies, it was suggested that the mechanism of action of the drug is associated with the inhibition of inflammatory mediators (cytokines and prostaglandins), stimulation of anabolic and slowdown of catabolic processes in the cartilage matrix [41,42].
High molecular weight GL [43], when comparing 3 weekly injections with NSAIDs or a combination of NSAIDs and GL, was more effective than NSAIDs, and can probably be used instead of or together with NSAIDs. In a retrospective study of 336 patients with gonarthrosis, a good response was observed in 76% [44], with the treatment effect maintaining an average of 8 months. Local adverse reactions were observed in 8% of patients, ranging from minor to the development of acute synovitis with effusion, requiring intra-articular corticosteroids or a short course of NSAIDs.
A comparative study of high- and low-molecular-weight GL in 70 patients with knee OA (3 weekly injections) revealed a higher effectiveness of the first drug, with an equally small number of adverse local reactions [45]. However, the use of high and low molecular weight GL requires further research to better determine the place of this therapy and especially to assess the effect of long-term use on slowing down or reversing the development of cartilage degradation. GL is currently defined as a disease-modifying agent and is recommended for patients who do not respond to other therapies or who cannot tolerate NSAIDs.
Confirming the structure-modifying properties of drugs in humans is extremely difficult, since OA is usually a very slowly progressive disease. It must be taken into account that it is possible that any remedy effective against the symptoms of a disease may potentially have structure-modifying properties. Likewise, it is known that any drug with structure-modifying properties has a small and, as a rule, delayed effect on the symptoms of the disease. In addition, methods for studying disease progression are limited, making clinical testing of structure-modifying drugs challenging. To demonstrate structure-modifying properties, clinical trials often use knee radiographs to assess OA outcomes; Methods are being developed that can assess the surface and volume of cartilage.
Drugs that modify symptoms of the disease have some advantage in assessing the effectiveness of OA treatment
. However, treating patients with OA is challenging. First, in a heterogeneous disease with an unpredictable course, such as OA, it is important to remember that treatment should not harm the patient. Secondly, it is difficult to create a unified treatment algorithm, since individual differences in the course of the disease are also great. Therefore, treatment of patients with OA should be considered taking into account an assessment of the benefits/risks of the therapy.
An important point at the beginning of treatment is the characterization of the pain syndrome in terms of the presence or absence of inflammatory changes. The assumption that a significant proportion of patients had only pain without signs of inflammation formed the basis for the creation of an algorithm for the treatment of patients with OA of the knee and hip joints, proposed by the American College of Rheumatology in 1995 [10,11]. If non-pharmacological treatments were insufficiently effective, analgesics (acetominophen 4.0 per day) were recommended as first-line drugs, followed by local therapy (except for hip OA), low doses of NSAIDs or other analgesics such as tramadol, and then therapeutic doses NSAIDs, intra-articular injections of glucocorticosteroids, hyaluronic acid, sulfated glycosaminoglycans. For patients at high risk of developing gastrointestinal complications, preventive treatment
misoprostol, proton pump inhibitors, high doses of H2-histamine receptor antagonists.
And although these recommendations have not lost their relevance, evidence is accumulating on the need for earlier prescription of anti-inflammatory therapy [12]. In addition, acetaminophen was not completely safe as reported in early studies, and Pincus et al. [2] noted the greater effectiveness of NSAIDs compared to simple analgesics.
Specific COX-2 inhibitors have a significantly higher benefit/risk ratio compared to traditional NSAIDs. These drugs appear to be considered as first-line drugs, particularly in patients with moderate to severe pain, with or without signs of inflammation, joint swelling and joint effusion. Patients with insufficient effectiveness and poor tolerability of these drugs should be treated with traditional NSAIDs with mandatory combination with preventive therapy, especially in those patients who have risk factors for the development of adverse events. It must be taken into account that most patients over 60 years of age have at least one risk factor for complications.
If there is a large effusion in the joint, the condition is significantly alleviated by intra-articular injection of steroids (injection into the hip joint is undesirable), but not more than 1-2 times a year.
The introduction of hyaluronic acid is especially indicated when treatment with analgesics and NSAIDs is ineffective, and there are also contraindications for their use.
Sulfated glycosaminoglycans may turn out to be very promising, not only because of their effectiveness and, possibly, structure-modifying effects, but also due to their good tolerability, which does not require medical monitoring, unlike taking NSAIDs.
Probably, the time of treating OA with monotherapy is becoming a thing of the past, and the expansion and deepening of knowledge about the pathogenesis of the disease, the mechanisms of action of drugs on different pathogenetic links of the disease allows us to hope that treatment regimens and new drugs will soon be developed that will be able to change the natural course of OA.
References:
1. Bradley ID, Brandt KD, Katz BP et al. Comparison of an antiinflammatory dose of ibuprofen. an analgesic dose of ibuprofen, and acetaminophen in the treatment of patients with osteoarthritis of the knee. N Engi J Med. 1991. 325:87–91.
2. Pincus T, Callahan LF, Wolfe F et al. Arthrotec compared to acetominophen (ACTA): clinical trial in patients with osteoarthritis (OA) of the hip or knee. Arthritis Rheum 1999. 42–.S404.
3. Towheed TE, Hochberg MC. A systematic review of randomized controlled trials of pharmacological therapy in osteoarthritis of the knee. Semin Arthritis Rheum 1997, 27:755–70.
4. Towheed TE, Hochberg MC. A systematic review of randomized controlled trials of pharmacological therapy in osteoarthritis of the hip. J Rheumatol 1997, 24:349–57.
5. Cryer B. Feldman M. Cyclooxygenase–1 and cyclooxygenase–2 selectivity of widely used nonsteroidal anti–inflammatory drugs. Am J Med 1998, 104:413–21.
6. Simon LS. Mffls JA. Drug therapy: nonsteroidal antiinflammatory drugs. N Engi J Med. 1980, 302:1179–85,1237–43.
7. Schiodt FV, Rjehling FA, Casey DL, Lee WM. Acetominophen toxicity m an urban country hospital. N Engi J Med. 1997, 337:1112–17.
8. Phiffips AC, Simon LS. NSATDs and the elders: toxicity and the economic implications. Drugs Aging 1997, 10:119–30.
9. Simon LS, Stand V. Clinical response to nonsteroidal antiinflammatory drugs. Arthritis Rheum 1997, 40:1940–43.
10. Hochberg MC, Altaian RD, Brandt KD et al. Guidelines for medical management of osteoarthritis. L Osteoarthritis of the hip. Arthritis Rheum 1995, 38:1535–40.
11. Hochberg MC, Altman RD, Brandt KD et al. Guidelines for medical management of osteoarthritis. P. Osteoarthritis of the knee. Arthritis Rheum 1995, 38:1541–46.
12. Altman RD, Hochberg MC, Moskowitz RW, Schnitzer TJ. Recommendations for the medical management of osteoarthritis of me and knee. Arthritis Rheum 2000, 43:1905–15.
13. Wolfe MM, Lichtenstein DR, Singh G. Gastrointestinal toxicity of me nonsteroidal antiinflammatory drugs. N Engi J Med. 1999, 340:1888–99.
14. Crofford LJ, Lipsky PE, Brooks P et al. Basic biology and clinical application of cyclooxygenase–2. Arthritis Rheum 2000, 43:4–13.
15. Lipsky PE. Abramson SB, Croiford LJ et al. The classification of cyclooxygenase inhibitors. J Rheumatol 1998, 25:2298–2303.
16. Laine L, Haper S, Simon T et al. A randomized trial comparing the effect ofrofecoxib, a cyclooxygenase–2 specific inhibitor, with that of ibuprofen on gastroduodenal mucosa of patients with osteoarthritis. Gastroenterology 1999, 117:776–83.
17. Hawkey CJ. COX-2 inhibitors. Lancet 1999, 353:307–14.
18. Bensen WG. Flechthner JJ, McMillen L et al. Treatment of osteoarthritis with celecoxib, a cyclooxygenase–2 specific inhibitor: a randomized controlled trial. Mayo Clin Proc 1999, 74:1095–1105.
19. Reginster J, Bias P, Buchner A. First clinical results of licofelone (ML3000), an inhibitor of COX–1, COX–2 and %–LOX, for the treatment of osteoarthritis. Ann Rheum Dis 2002. 61 (Suppl):THU0189,pll6.
20. Pelletier IP. The influence of tissue cross–talking on OA progression: role of nonsteroidal antiinflammatoly drugs. Osteoarthritis Cartilage 1999, 7:374–6.
21. Garcia Rodriguerz LA. Nonsteroidal antiflammatory drugs, ulcers and risk: a collaborative meta–analysis. Semin Arthritis Rheum 1997, 26(suppl):16–20.
22. Simon LS, Hatoum HT, Bittman RM et al. Risk factors for serious nonsteroidal–mduced gastrointestinal complications: regression analysis of the MUCOSA trial. Fam Med 1996. 28:202–208.
23. Wilcox CM, Alexander LN, Cotsonis GA, Clark WS. Nonsteroidal antiinflammatory drugs are associated with both upper and lower gastrointestmal bleeding. Dig Dis Sci 1997, 42:990–97.
24. Schnitzer TJ, Kaniin N, Olson WH. Tramadol allows reduction of naproxen dose among patients with naproxen–responsive osteoarthritis pain: a randomized, double blind, placebo controlled trial. Arthritis Rheum 1999, 42:1370–77.
25. Gallacchi G, Marcolongo R. Pharmacokmetics of diclofenac hydroxyethylpyrrolidine plasters in patients with monolateral knee joint effusion. Drugs Exp Clin Res 1993: 19:95–7.
26. Moore RA, Tramer MR, Carroll D et al. Quantitative systematic review of topically applied non-steroidal anti-inflammatory drugs. BMJ 1998, 316:333–38.
27. Uebelhart D, Thonar E, Zhang J, Williams J. Protective effect of exogenous 4.6–sulfate in the acute degradation of articular cartilage in the rabbits/ Osteoarthritis Cartilage 1998, 6 (supplement A):6–13.
28. Morreale P, Manopulo R, Galati M et al. Comparison of the anti–inflammatory efficacy of chondroitin–sulfate and diclofenac sodium in patients with knee osteoarthritis. J Rheumatol 1996.23:1385–91.
29. Uebelhart D, Knussel 0, Theiler R. Efficacy and tolerance of oral chondroitin-sulfate in painful knee osteoarthritis: a double-blind, placebo-controlled, multicentre 6-month trial. Osteoarthritis Cartilage, 1999.7, Suppi A, abstr 144.
30. Alekseeva L.I., Benevolenskaya L.I., Nasonov E.L., Chichasova N.V., Karyakin A.N.. Structum (chondroitin sulfate) - a new drug for the treatment of osteozrthrosis (O A) // Ter . Archive 1999, No. 5, p. 51–53.
31. Leeb BF, Schweitzer H, Montag K, Smolen JS. A meta-analysis of chondroitmsulfate m the treatment of osteoarthritis. Osteoarthritis Cartilage, 1999, 7, Suppi A. abstr 130.
32. Eugenio-Sarmiento RM, Manapat BHD, Salido EO, The efficacy of chondroitin-sulfate in the treatment of knee osteoarthritis: a meta-analysis. Osteoarthritis Cartilage, 1999, 7, Suppi A, abstr 139.
33. Uebelhart, Thonar E, Delmas P et al. Effects of oral chondroitin sulfate on the progression of knee osteoarthritis: a pilot study. Osteoarthritis Cartilage 1998; 6 (suppl A): 39–46.
34. Michel BA, Bruhlmann P, Stuck! G, Uebelhart D. Chondroprotection through Chondrosutf: the Zurich study. Ann Rheum Dis 2002, 61 (Supp I): 116.
35. Muller–Fasbender H, Bach G, Haase W et al. Glucosamine sulfate compared to ibuprofen m osteoarthritis of the knee. Osteoarthritis Cartilage 1994;2:61–69.
36. Noack W, Fisher M, Forster KK et al. Glucosamine sulfate in osteoarthritis of the knee. Osteoarthritis Cartilage 1994;2:51–59.
37. Register J–Y, Deroisy R, Paul I et al. Glucosamine sulfate significantly reduced progression of knee osteoarthritis over 3 years: a large, randomized, placebo-controlled, double-blind, prospective trial. Arthritis Rheum 1999, 42 (suppl):S402.
38. Leffler CN, Phiffipi AF, Lefner SG et al. Glucosamine, chondroitin and magnesium ascorbate for degenerative joint disease of the knee or low back pain: a randomized, double–blind, placebo–controlled pilot study. Mil Med 1999, 164:85–91.
39. Shanklad WE, The effects of glucosamine and chondroitin sulfate on osteoarthritis of the TMJ: a preliminary report of 50 patients. Cranio 1998, 16:230–235.
40. Balazs EA, Denlinger JL. Viscosupplementation: a new concept in the treatment of OA. J Rheumatol 1993, Suppl39:3–9.
41. Yasui T, Akatsuka M, Tobetto K et al. The effect ofhyaluronan on interleukin–2 induced prostaglandin–E2 production in human osteoarthritis svnovial cells. Agents Action 1992, 37:155–6.
42. Ghosh P. The role of hyaluronic acid (hyaluronan) m health and disease: interactions with cells, cartilage and components of the synovial fluid. Clin Exp Rheumatol 1994, 12:75–82.
43. Adams ME, Atkinson MH, et al. The role of Viscosupplementation with hylan G–F 20 (Synvisc) in the treatment of osteoarthritis of the knee: a Canadian multicentre trial comparing hylan G–F 20 alone, hylan G–F 20 with non–steroidal anti–inflammatory drugs (NSAIDs) and NSATOs alone. Osteoarthritis Cartilage 1995, 3:213^225.
44. Lussier AJ, Cividino AA, McFariane CA et al. Viscosupplementanon with hylan for the treatment of osteoarthritis: findings from clinical practice in Canada. J Rheumatol 1996, 23:1579–1585.
45. Wobig M, Bach G, Beks P, et al. The role of elastoviscosity in the efficacy of viscosupplementation for osteoarthritis of the knee: a comparison of hylan G–F 20 and a lower–molecular–weight hyaluronan. Clin Ther, 1999 Sep; 21(9): 1549–1562.
Gonarthrosis of the knee joint: symptoms and stages. What's happening to you?
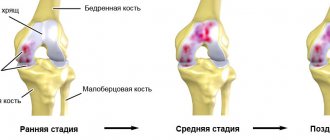
Stage 1 gonarthrosis.
The process of arthrosis goes unnoticed, but makes itself felt when there is little left of the cartilage. At first the pain is mild and infrequent, especially if you get up in the morning or sit for a long time, and then get up and go. These are “starting pains” - a typical sign of stage 1 gonarthrosis.
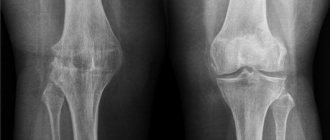
Stage 2 gonarthrosis.
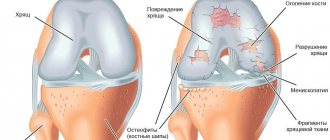
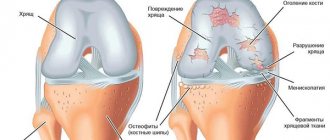
It hurts more often and more severely here. Stand on your feet for a long time, or lift something heavy, and a couple of unpleasant hours are guaranteed. And also morning stiffness, crunching, pain when going down the stairs, and, of course, joints hurt “due to the weather.” The cartilage in the joint is further destroyed, the bones themselves suffer, their articular surfaces are flattened and protruded, and “spikes”—osteophytes—appear. They injure the joint lining and cause pain.
Stage 3 gonarthrosis.
The joints become deformed and swollen, and the legs bend into an O- or X-shape. The joint often “jams” - in a certain position it is impossible to bend or straighten it. This occurs when pieces of cartilage break off and become lodged between bones. The pain becomes severe, constant and disrupts overall well-being. Walking is difficult and painful, even with a cane. Inflammation intensifies - there is more fluid, movements are limited up to contracture and complete cessation of movement in the joint, temperature and ESR rise.
Why do most patients improve from the first course of treatment?
We monitor the qualifications of specialists.
Our doctors have deep knowledge in diagnostics, neurology, and therapy. This way, it is possible to select the right combination of procedures, relieve the drug burden on the body, and influence both the symptoms and the cause of the disease. The average work experience of our doctors is more than 20 years.
We take your condition into account.
Before treatment is prescribed, you will be thoroughly examined. We consider the body as a whole and look for the root cause of the disease. If you have already been examined in other clinics, take the results with you. The doctor will draw up a personal treatment and rehabilitation plan so that gonarthrosis does not bother you anymore.
We provide truthful information in a language you can understand.
The doctor is not trying to scare. His task is to find out what is wrong and select the most effective treatment. Therefore, during the consultation, he asks you in detail about your symptoms, treatment and its results, concomitant diseases, lifestyle and eating habits. The doctor clearly explains what he will do and why. And if you have questions, you can always contact him and ask them.
Perhaps you are still in doubt. You have a choice - wait until surgery is needed, endure pain and restrictions. Or treat gonarthrosis with safe and proven methods now. Call 8 (495) 153-52-14 and make an appointment.
Diagnosis of gonarthrosis 2 degrees
Modern medicine offers quite a few methods for the treatment of grade 2 knee joint gonarthrosis and its accurate diagnosis. We know how important it is to make the correct diagnosis. Therefore, for greater efficiency, we suggest acting comprehensively.
If bilateral gonarthrosis of the knee joint of the 2nd degree is diagnosed, then the doctor himself determines whether treatment will be carried out in parallel or alternately, with a break of several weeks.
Today, the diagnosis of grade 2 gonarthrosis includes several stages.
Examination of a patient by an orthopedist
This is a basic and very important condition. A good specialist will palpate the patient’s joints, take linear measurements of his bones, and also determine the degree of mobility of the diseased joint from different angles.
If gonarthrosis is suspected, the doctor will prescribe the following instrumental diagnostic methods.
Clinical researches
These are tests that consist of:
- - ESR analysis;
- - fibrinogen level, as well as other biochemical test data.
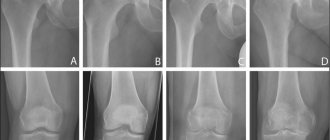
X-ray
The undoubted diagnostic leader in the treatment of grade 2 knee joint gonarthrosis is x-ray. If at first this method is not very informative, then at a later stage it will turn out to be very effective.
At this time, the x-ray shows narrowing of the joint spaces, thinning of the bone tissue, noticeable hardening of the tissue under the cartilage, noticeable bone damage, ossification of the cartilage tissue, and salt deposition.
In this case, the formation of osteophytes is very possible. These are so-called bone growths formed around cartilage.
Ultrasound
An ultrasound examination of grade 2 gonarthrosis will show the most accurate results, but it certainly cannot replace an x-ray.
Therefore, it is so important that all patients with gonarthrosis have an X-ray on their hands.
MRI
Magnetic resonance imaging is a very advanced diagnostic method today. This method allows you to accurately, in detail study and assess the degree of damage to all parts of the joint, and determine changes that have occurred in the cartilage tissue.
The obvious advantage of MRI is the very high accuracy of the method. Because very often the patient’s gonarthrosis occurs in conjunction with other diseases of the musculoskeletal system.
However, this method also has its drawback - it is quite expensive.
Tests for coronavirus
- Test for coronavirus
- Coronavirus test for organizations
- Testing for coronavirus at home
- Testing for coronavirus at home in 12 hours!
- Testing for coronavirus in Lyubertsy in 12 hours!
- Testing for coronavirus in Nekrasovka in 12 hours!
- Testing for coronavirus in Korolev in 12 hours!
- Test for coronavirus on the Sokol metro station
- Coronavirus test at Kolomenskaya metro station
- Coronavirus test at Voykovskaya metro station
- Test for coronavirus in Nekrasovka
- Coronavirus test in Korolev
- Test for coronavirus in Lyubertsy
- Test for coronavirus in Mytishchi
- Test for coronavirus at home Mytishchi
- Test for coronavirus at home Korolev
- Test for coronavirus at home Lyubertsy
- Test for coronavirus at home Nekrasovka
Any tests can be taken at clinics in the East Clinic network.