Preparations Dona and Aflutop are designed to protect the cells of the cartilage layer and restore them. They help fix calcium and phosphorus in the bones and retain water in the joint. The effectiveness of the therapeutic effect is influenced by the severity of the disease and the individual characteristics of the body.
Preparations Dona and Aflutop are designed to protect the cells of the cartilage layer and restore them.
Characteristics of Don
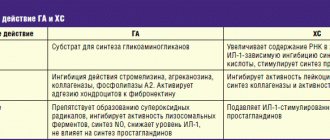
The active component of the drug is glucosamine. It restores damaged cartilage tissue, having a positive effect on its trophism. The substance is a monosaccharide with a low molecular weight, so it easily penetrates cell membranes.
Its action is explained by its participation in the biochemical process of the addition of glucose residues to protein and lipid molecules. The result is glycosaminoglycans, which are part of the connective tissue matrix of cartilage. The substances enter the intercellular fluid in bone tissue; they maintain the normal volume of synovial fluid.
The medication has analgesic and anti-inflammatory effects. The medicine stimulates the production of collagen and elastin, preventing destructive phenomena in the joints.
Degenerative processes in joints are associated with the activity of the enzymes phospholipase A2 and collagenase. These enzymes break peptide bonds in collagen and hydrolyze lipid molecules. As a result, the cell membrane and cellular structures are destroyed. These pathological changes lead to the onset of the inflammatory process. The active ingredient of the drug blocks the work of enzymes, thereby preventing the development of inflammation and destructive changes in the joint.
Dona restores metabolic processes and promotes the absorption of nutrients and oxygen by tissues.
Glucosamine promotes the binding of sulfur and the formation of esters with this element, which helps maintain normal elasticity of the cartilage layer. The substance promotes the fixation of water molecules in connective tissue and increases the permeability of the joint capsule. The drug restores metabolic processes and promotes the absorption of nutrients and oxygen by tissues. Thanks to the medicine, calcium and phosphorus ions are well absorbed from the blood, ensuring bone strength.
Introduction
The continuing interest in osteoarthritis (OA) is dictated primarily by its widespread prevalence and progressive nature, leading to premature disability.
OA affects more than 300 million people in 195 countries, and there is an increase in the period of time patients live with disability (YLDs – Years lived with disability) due to OA [1]. In Russia, 13% of the population suffers from OA. OA most often affects weight-bearing joints: the knees, hips, as well as the joints of the hands and spine. As the disease progresses, due to constant pain and limited functional ability of the joints, the quality of life of patients decreases, and limited physical capabilities often lead to disability, which remains an important social problem [2, 3]. OA is a disease with high comorbidity and is most often combined with obesity, diabetes mellitus (DM), arterial hypertension (AH) and other cardiovascular diseases (coronary heart disease, atherosclerosis) [4]. The term “comorbidity” (Latin co – together, morbus – disease) was proposed to be used in 1970 by A. Feinsten, a doctor, researcher and epidemiologist [5]. A fundamental clarification of this term was given by HC Kraemer and M. Akker in 1995; they defined it as a combination of several chronic diseases in one patient [6, 7]. The strategic tactics of medicine is to develop new and improve existing methods for treating diseases. OA therapy should be aimed not only at reducing pain and improving the functional state of joints, but also at slowing down the progression of OA. Reduction of pain in most cases is achieved through symptomatic therapy with nonsteroidal anti-inflammatory drugs (NSAIDs). However, their use, often long-term, is associated with a high risk of developing adverse events (AEs) from the gastrointestinal tract, cardiovascular system, kidneys and other organs, which is especially important for comorbid diseases in patients receiving drugs to treat these diseases . In this regard, there is a need to take into account drug interactions and take a differentiated approach to prescribing NSAIDs to patients with OA, taking into account risk factors for possible complications associated with their use. From this point of view, therapy with drugs from another group that have both symptom-modifying and structure-modifying properties and have a high safety profile (SYSADOA - symptomatic slow acting drugs for osteoarthritis) seems relevant. Indeed, in 2014, the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis ESCEO (European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases) included SYSADOA in a step-by-step algorithm for the treatment of knee OA as first-line drugs [8 ]. According to the recommendations of the Association of Rheumatologists of Russia, the Federal Clinical Guidelines for the Treatment of OA, and the consensus of experts on OA, drugs in this group are classified as basic treatments for OA, which should be prescribed immediately after diagnosis.
SYSADOA have, on the one hand, a symptomatic effect, i.e. reduce pain and improve joint function, on the other hand, some of them can slow down the progression of OA [9]. This group includes drugs belonging to substances of different chemical structures: glucosamine, chondroitin sulfate, unsaponifiable compounds of soy and avocado, diacerein, hyaluronic acid preparations.
Unlike NSAIDs, they have a slow onset of action, often up to 8–12 weeks, but unlike symptomatic drugs, the effect after a course of use lasts up to 2–4 months after the end of therapy. Drugs in this group have a potential structure-modifying effect and high safety. And although the opinion on these drugs is still controversial, data from meta-analyses and many clinical studies have demonstrated a reduction in the dose of NSAIDs used during the use of SYSADOA, which is extremely important for patients with OA and comorbidity [10].
The results of clinical studies performed in our country indicate that with parenteral administration of SYSADOA, their analgesic effect develops much faster (on average after 1–1.5 months) compared to oral drugs (after 8–12 weeks of treatment). One of the representatives of this group of parenteral drugs, used in rheumatological practice for more than 20 years, is Alflutop. The drug is a standardized sterile bioactive concentrate from 4 types of small sea fish.
It contains sulfated glycosaminoglycans (GAGs), similar to the matrix of hyaline cartilage: chondroitin-4 sulfate, chondroitin-6 sulfate, dermatan sulfate, keratan sulfate, low molecular weight polypeptides, free amino acids and trace elements (Na, K, Ca, Mg, Fe, Cu, Zn ), which are important for metabolic processes and the metabolism of connective tissue in general. The mechanism of action of the drug Alflutop is to suppress the production of a number of pro-inflammatory mediators (in particular, interleukins IL-6, IL-8 and IL-1β), matrix metalloproteinases (ADAMTS4, MMP13, MMP3), inhibition of angiogenesis associated with the production of vascular endothelial growth factor ( VEGF - Vascular endothelial growth factor), as well as stimulation of chondrogenesis (effect on the extracellular release of TGF-β - Transforming growth factor β, and expression of the SOX-9 gene) [11]. In addition, the drug also has an antioxidant effect due to an increase in catalase activity and a decrease in the level of intracellular superoxide anion/hydrogen peroxide [12].
Alflutop has a multicomponent effect: it affects the metabolism of chondrocytes, stimulates the synthesis of matrix macromolecules, has antioxidant activity, in addition, the key point of its action is the combination of antihyaluronidase activity and stimulation of the synthesis of hyaluronic acid (HA). The drug can be administered both intramuscularly (i.m.) and intra-articularly (i.a.); to obtain a faster effect, the methods of drug administration can be combined.
Drugs of the SYSADOA group are quite effective and have a high safety profile, however, only some of the parenteral forms of this group meet the requirements of evidence-based medicine [13, 14]. Thus, convincing data were obtained in our previously conducted multicenter, double-blind, placebo-controlled study to evaluate the symptomatic and structural-modifying effects of the drug Alflutop.
The two-year study involved 90 patients randomized into 2 groups, one of which (n=45) received intramuscular injections of Alflutop 1 ml in courses of 20 days at 6-month intervals (4 courses in total), the second (n=45) ) – placebo (injections of 0.9% NaCl solution) according to the same scheme. The symptom-modifying effect was assessed by the dynamics of pain, the WOMAC index (Western Ontario and McMaster Universities Arthrose index), EQ-5D (EuroQoL-5D), general health status using a visual analogue scale (VAS) and assessment of the effectiveness of treatment by the doctor/patient, response to therapy according to the OMERACT-OARSI criterion.
In order to study the structural-modifying effect of Alflutop, all patients initially and after 2 years underwent magnetic resonance imaging (MRI) of the target joint, as well as radiography of the knee joints in a standing position using a position frame. The dynamics of radiological changes were assessed in points: by the width of the medial gap of the knee joint and the presence of osteophytes of the femur and tibia. In this study, biochemical markers of cartilage tissue degradation (CTX-II and COMP) were also determined at the beginning of the study, after 3 months and at the end of observation. The results of the study showed a significant reduction in pain, stiffness and improvement in the functional state of joints in the main group compared to placebo (p<0.005). The results of this multicenter, double-blind, placebo-controlled study confirmed the structure-modifying effect of Alflutop. MRI data were obtained showing a reduction in the progression of OA in the group receiving Alflutop (slowing the narrowing of the joint space and slowing the increase in osteophyte size) of the knee joints compared with placebo (odds ratio [OR] -1.5; 95% confidence interval [CI] - 1.17–1.99; p<0.003). It should be noted that in the first group, already 3 months from the start of therapy, there was a tendency towards a decrease in the content of the marker of cartilage tissue degradation - CTX-II, while in the placebo group there was a slight increase in both CTX-II and COMP, which indirectly indicated about the progression of the disease.
The use of Alflutop is not limited to OA; it is widely and successfully used in vertebrology. Its effectiveness and safety have been proven by O.S. Levin et al. in a double-blind, placebo-controlled study of 83 patients with chronic vertebrogenic lumbar sciatica [15]. Side effects with Alflutop (pain at the injection site, headache, dizziness) did not occur more often than with placebo.
Later, in 2008, O.S. Levin et al. presented the results of an open multicenter study to evaluate the effectiveness and safety of Alflutop in patients with vertebrogenic cervicobrachialgia. Alflutop was administered intramuscularly No. 20 to 149 patients, 60 patients formed the control group [16]. The study demonstrated the ability of Alflutop to reliably reduce the severity of pain and improve mobility in the cervical spine and shoulder joint. There was also a faster positive trend in quality of life indicators than with placebo. AEs included pain at the injection site, headache, and dizziness. Side effects were mild and did not require discontinuation of the drug.
Purpose of the study: despite the presence of a large evidence base, including double-blind randomized controlled studies confirming the analgesic, anti-inflammatory and structure-modifying effects of Alflutop, in order to study options for increasing adherence to OA therapy, an open randomized multicenter study of the effectiveness and safety of using the drug Alflutop in an alternating mode was conducted (2.0 ml IM every other day for 20 days) compared with the standard (1.0 ml IM daily for 20 days) in patients with OA of the knee joints.
Methods
The study included 130 patients, 65 patients in each group. The duration of the study was 3 months. The study groups included patients aged from 41 to 74 years, the average duration of the disease was 4 years (from 0 to 22 years) and the average body mass index (BMI) corresponded to excess weight (Table 1).
Most patients had radiographic stage II of knee OA according to Kellgren-Lawrence (75.4% in the first group and 70.8% in the second). A comparative analysis established the homogeneity of the groups in terms of the frequency of concomitant diseases, identified in 96.9% of cases. Thus, hypertension in patients of the first group was diagnosed in 70.8% of cases, in patients of the second group - in 67.7%, dyslipidemia - in 50.8 and 60%, type 2 diabetes - in 6.2 and 4.6%, respectively. (p>0.999).
The effectiveness of therapy was assessed according to generally accepted criteria: pain dynamics (according to VAS), WOMAC index indicators (pain, stiffness and functional impairment - FN), the value of the “get up and go” test, health status according to EQ-5D; by changes in scores on the Patient's Global Assessment (PGA) and Investigator's Global Assessment (IGA) scales, and by response to therapy according to OMERACT-OARSI criteria. Safety assessment in this study was based on the frequency of AEs and serious AEs of varying severity, according to subjective complaints, laboratory tests (complete blood count, urine test, biochemical blood test, which included determination of levels of glycated hemoglobin, glucose, total protein , alanine aminotransferase - ALT, aspartate aminotransferase - AST, bilirubin, creatinine, low-density lipoprotein cholesterol - LDL, high-density lipoprotein cholesterol - HDL, triglycerides, γ-glutamyltransferase - GGT, sodium, potassium, chlorides); glomerular filtration rate - GFR according to the Cockcroft-Gault formula, physical examination, assessment of vital signs and electrocardiography (ECG). All studies were carried out at the beginning and at the end of Alflutop therapy, as well as one month after the end of the study. The functioning of the hemocoagulation system was also monitored, blood clotting indicators were monitored: activated partial thromboplastin time (APTT), prothrombin time (PTT) in seconds and percentages, international normalized ratio (INR).
Statistical analysis was performed using the R software package (“The R Project for Statistical Computing”, https://www.r-project.org). All statistical tests were two-sided with a significance level of 5%; all confidence intervals (CIs) were two-sided 95%; Continuous data are represented by the number of observations, mean with standard deviation (SD), median (Me) and range; discrete quantities are described by absolute and relative frequencies.
results
The results of the study showed that patients in both groups had a statistically significant decrease in pain in the knee joints when walking already by the 20–21st day of therapy (B3) (Table 2).
The change in Me in the first group was -25.0 mm, in the second - -32.0 mm (p<0.001). A further reduction in pain was observed within a month after completion of treatment (B4) (Me: -36.0 and -40.0 mm, respectively; p<0.001), indicating an aftereffect of the drug (Fig. 1).
Analysis of pain, stiffness, and physical function according to WOMAC revealed a similar pattern (see Table 2).
A significant decrease in the analyzed parameters was also observed at the end of therapy (B3). The change in median pain according to WOMAC by the 20–21st day of treatment was -18.8 mm in the group of patients receiving Alflutop in an alternating regimen, and 2.8 mm in the group of standard drug administration (p<0.001); stiffness – -15.0 and -18.0 mm, respectively (p<0.001); FN – -16.6 and -14.2 mm, respectively (p<0.001). After 2 months from the start of therapy, there was a further decrease in pain (Me: -21.8 mm in the first and -28 mm in the second group; p<0.001), stiffness (Me: -21.5 and -25 mm, respectively; p<0.001), improvement in functional ability (Me: -22.1 and -23.1 mm, respectively; p<0.001, Fig. 2–4).
Improvement in quality of life according to EQ-5D was noted in both groups of patients throughout the entire observation period (Table 1–2, Fig. 5). Indicators of general health assessment (VAS) also indicate the effectiveness of the therapy. Thus, compared with the initial values for B3, Me increased by +20.0 mm in the alternating treatment group and by +19.0 with standard therapy (p<0.001); to B4 – by +23.0 and +22.0 mm, respectively (p<0.001, Table 1–2, Fig. 6).
Data from the OMERACT-OARSI index, which recorded a high percentage of response to therapy in both groups, deserve special attention. Thus, by the end of treatment, 70.8% of patients in the first and 75.4% of the second groups were responders. By the end of the observation period, the number of patients classified as responders to therapy increased - 84.6 and 81.5%, respectively (Fig. 7).
Thus, the results of a multicenter clinical study showed that both treatment regimens used have comparable therapeutic potential, having a positive effect on the clinical manifestations of OA.
There were no SAEs recorded in both groups during treatment and observation. In the Alflutop 1 ml IM therapy group daily (first group), 10 (15.4%) AEs were recorded, in the Alflutop 2 ml IM therapy group every other day (second group) - 19 (29.2%) AEs.
Table 3 contains general information about AEs.
In the treatment group Alflutop 1 ml IM daily, 8 AEs in 4 patients were of mild severity and 2 AEs in another 2 patients were of moderate severity. All were unrelated to the study drug (IP). The most common AEs are increased blood pressure and headache. By the end of the study, all AEs resolved.
In the Alflutop 2 ml IM therapy group every other day, 19 AEs developed in 12 patients: 18 AEs were mild and 1 AE was moderate, 17 of them were not related to PV, and 2 AEs were unlikely to be related to PV. therefore, all 19 AEs were classified as non-PI AEs. Among AEs in this group, acute respiratory viral infection was observed in 4 patients, cystitis – in 1; 3 patients had arthralgia, 1 had joint swelling, and another 1 had pain in the spine. An increase in blood pressure was observed in 1 patient, and in another 1 patient there was a clinically insignificant (CI) increase in ALT levels. Epigastric pain was recorded in 1 patient; one patient each reported dyspepsia and epigastric discomfort. By the end of the study, 18 AEs resolved completely and only 1 AE resolved partially. According to the ECG data, in 12 leads the patients had only CNC abnormalities. Such deviations were observed in 39 (60.0%) patients in the Alflutop 1 ml IM therapy group daily and 33 (50.8%) patients in the Alflutop 2 ml IM therapy group every other day.
The following indicators were analyzed: hemoglobin, hematocrit, erythrocytes, leukocytes and blood count, platelets, ESR according to Westegren. Initially, patients in two groups had CNC deviations in some indicators, but at the end and follow-up visits their indicators normalized; other patients who had normal indicators at the initial visit had CNC deviations. In patients who did not suffer from diabetes in both groups during Alflutop therapy, no clinically significant increase in glucose was observed. Slightly elevated glucose levels in some cases were apparently associated with improper blood donation (not on an empty stomach). In patients with diabetes in both groups, CNC fluctuations in glucose levels were observed, but there was no tendency to increase glycemia.
Indicators of biochemical tests: total protein, sodium, potassium, chlorides, ALT, AST, total bilirubin, creatinine, GGT, by the end of the study in both groups had an insignificant percentage of CNC deviations. There were no clinically significant deviations in renal function indicators (urea, creatinine, general urinalysis).
Conclusion
Our research results confirm the high efficiency and safety of Alflutop both with standard and with alternating regimens of use. A rapid, comparable analgesic effect was demonstrated in both study groups. During treatment, a significant improvement in the function of the knee joints and quality of life was noted. In general, the safety profile of the drug can be characterized as favorable. No serious or severe AEs were identified during the study.
In all patients, vital signs did not have clinically significant deviations throughout the study.
The study allows us to positively evaluate the possibility of using the drug Alflutop not only daily IM 1 ml No. 20, but also 2 ml No. 10 IM every other day by patients with knee OA, which may help increase patient adherence to therapy. Treatment with Alflutop is safe, well tolerated by patients, does not have a negative effect on the functions of vital organs and systems, and therefore can be used in complex therapy of patients with OA with comorbidity.
Transparency and independence of research. The strength of this work is that it was conducted and supervised by an independent contract research organization strictly in accordance with applicable laws and applicable regulatory requirements.
Characteristics of Alflutop
Active substances – chondroitin sulfates. They regulate the growth and development of cartilage tissue. These are protein components containing sugar residues and sulfur atoms. These complex compounds are the adhesive and lubricating substance that ensures the normal functioning of the joint. The ingredients of the drug increase the permeability of the cell membrane to macro- and microelements.
By making the necessary chemical elements highly bioavailable, the drug has a positive effect on regenerative processes.
It inhibits the degradation and atrophy of connective tissue, prevents the thinning of the cartilage layer, and accelerates the restoration of motor activity of the joint. Chondroitin sulfates of the drug prevent the appearance of tumors and thorns that cause pain when moving.
The active substance inhibits lysosome enzymes that hydrolyze macromolecules. By preventing the destruction of bonds between amino acids, they inhibit damaging processes in connective tissue. In addition, they prevent loss of moisture from the cartilage layer and, as a result, premature wear and deformation of the joint. The medicine provides good blood supply to the joint, prevents platelet aggregation and the formation of blood clots in the vessels surrounding the joint capsule. The substance stimulates the development of a network of capillaries around the joint.
Alflutop ensures good blood supply to the joint, prevents platelet aggregation and the formation of blood clots in the vessels surrounding the joint capsule.
Comparison of Dona and Alflutop
Both drugs have chondroprotective properties. Having a similar composition, they have an analgesic and anti-inflammatory effect that is similar in effectiveness and efficiency. By increasing the strength of connective tissue, they help smooth the joint, soften shock loads, and prevent joint destruction.
Similarities
Both medications correct metabolic processes in bone and cartilage tissues and quite effectively eliminate pain. The drugs can be considered analogues, since the active ingredients of both drugs are identical. They consist of mucopolysaccharides and participate in the formation of chondroitinsulfuric acids.
Medicines equally affect water-salt metabolism, bind water in all tissues of the joint, regulate the diffusion of cations and optimize intercellular interaction. The active substances of both drugs increase the viscosity of connective tissue, resulting in easy sliding of the articular surfaces.
Both drugs promote the deposition of calcium cations in the bones, providing a preventive effect in the fight against osteoporosis.
Dona and Alflutop treat arthrosis and arthritis equally well, and regulate the amount of synovial fluid in the joint. Both drugs are well absorbed and are excreted fairly quickly in the urine and feces.
What is the difference ?
The drugs differ in the sources of raw materials necessary for their production. Unlike Dona, Alflutop is made from natural raw materials - processed cartilage and bone tissue of small sea fish. Therefore, the drug is available only in the form of a solution containing a concentrated active ingredient.
Dona has several dosage forms:
- powder;
- capsules;
- solution for intramuscular administration.
Various pharmacological forms have led to a more extensive list of contraindications for the use of Dona. This drug can be used from the age of 12 years, while the use of Alflutop is permitted no earlier than 18 years.
To achieve a therapeutic effect, Alflutop is indicated for injection into the diseased joint, which is a painful procedure. This imposes restrictions on the use of the product by persons with a low pain threshold. Taking Dona is not associated with such pain, you can choose a convenient option for use.
What is more effective?
Clinical studies have shown that the end result from the use of Dona and Alflutop is identical. Relief occurs faster when using Alflutop, especially in patients with acute pain. This may be explained by the fact that the active component is injected directly into the diseased joint.
When Dona is administered intramuscularly, the medicine does not penetrate into the diseased joint so quickly, because first it must enter the bloodstream.
Taking Dona may be associated with the passage of the drug through the digestive tract. Because of this, the medicinal substance is partially destroyed by digestive juices, and the time it takes for the medicine to reach the painful site increases.
Which is cheaper?
Since Alflutop is made from natural raw materials, the cost of this drug is higher than that of Don's medicine. To produce the latter, synthetic or artificial chemical compounds are used, so the price is lower and the availability of the drug is higher.
Results and discussion
When analyzing the clinical picture of the disease, syndromes of cervicalgia, lumbodynia, and radiculopathy at the lumbosacral level were identified in most patients.
When assessing pain, a significant decrease in the severity of pain was noted over 5 years in all study groups, but especially in the 1st group. Thus, in group 1 pain was assessed:
— by 8 points in 2013, 11 people, in 2014, 1 patient, in the next 3 years, not a single patient had 8 points on the VAS scale;
— by 7 points in 2013 13 patients, in 2014 6, in 2015 3, in 2021 2; in 2021, this level of pain was not noted in any patient;
— pain was rated 6 points in 2013 by 7 patients, in 2014 by 18, in 2015 by 13, in 2021 by 12, in 2021 by 3.
It is worth noting that in 2021, 20 patients already rated pain on the VAS as less than 5 points (Fig. 1)
.
Rice.
1. Dynamics of pain severity according to VAS in group 1. Thus, during the observation period, there was a redistribution of patients from groups with severe pain syndrome (6-8 points) in favor of groups with less severe pain syndrome (less than 5 points).
In group 2 (intramuscular administration of Alflutop) there was also a decrease in the severity of pain during observation:
— 6 patients assessed pain as 8 points in 2013, 2 in 2014; in subsequent years, no one had 8 points on the VAS scale;
— pain syndrome was reported as 7 points in 2013 by 36 patients, in 2014 7 points were recorded in 34 people; in 2015 - 30, in 2016 - 18; in 2021, a pain level of 7 points was not noted in any patient;
— pain was rated 6 points in 2013 by 14 people, in 2014 by 20, in 2015 by 20, in 2021 by 26, in 2021 by 26;
— pain was rated below 5 points in 2015 by 6 patients.
It is noted that in 2021, already 13 people rated their pain as less than 5 points (Fig. 2)
.
Rice.
2. Dynamics of pain severity according to VAS in group 2. Thus, in group 2 there was also a redistribution of patients from groups with severe pain syndrome (6-8 points) in favor of groups with less severe pain syndrome (less than 5 points).
It is also worth noting that in the group of patients in whom Alflutop was administered paravertebrally (group 1), the average pain score in 2013 was 7.1 points, in 2014 - 6.1 points, in 2015 - 5. 9 points, in 2021 - 5.3 points, in 2021 - 5.1 points, i.e. there was a decrease in the average severity of pain within 3 points.
In the group of patients who received Alflutop intramuscularly, the average pain score in 2013 was 6.9 points, in 2014 - 6.7 points, in 2015 - 6.4 points, in 2021 - 6.1 points , in 2021 - 5.2 points, i.e. there was a decrease in the average severity of pain within 1 point (Fig. 3)
.
Rice.
3. Dynamics of the average pain value according to VAS by group. When assessing the number of exacerbations of back pain in patients of both groups, a tendency to decrease was revealed throughout all years of observation.
Thus, in group 1, the largest number of exacerbations (6) per year in 1 patient was registered in 2013 in 3 patients. In subsequent years, such a frequency of exacerbations was not recorded in anyone. In the same year, 4 exacerbations were registered in 17 patients, 3 in 11. In 2014, the largest number of exacerbations (5) were in 4 patients, 4 in 15, 3 in 10, 1 in 2. In 2015 d. 5 exacerbations were registered in 1 patient, 4 in 10, 3 in 14, 2 in 5; 1 patient had no exacerbations within a year. In 2021, the largest number of exacerbations (4) was recorded in 2 people, 3 in 19, 2 in 10. In 2017, the largest number of exacerbations (3) was registered in 7 patients, 2 in 16, 1 at 8 ( Fig. 4)
.
Rice.
4. Dynamics of the frequency of exacerbations per year in group 1. Thus, the average number of exacerbations per year in the group of patients in whom Alflutop was administered paravertebrally was 3.8 cases in 2013, 3.6 in 2014, 3.1 in 2015, 2.7 in 2021 , in 2017 1.9; i.e., it has decreased by almost 2 times over 5 years.
In the 2nd group of patients, the largest number of exacerbations per year (6 exacerbations) was registered in 2013 in 26 patients. In the same year, 11 patients had 5 exacerbations, 8 had 4, 11 had 3. In 2014, 12 patients had 6 exacerbations, 23 had 5, 15 had 4, 3 had 3, 2 had 3. In 2015, 6 exacerbations were registered in 10 patients, 5 in 24, 4 in 11, 3 in 8, 2 in 3. In 2021, 6 exacerbations were in 7 people, 5 in 18, 4 — in 21, 3 — in 7, 2 — in 3. In 2021, 6 exacerbations were in 4 patients, 5 — in 9, 4 — in 27, 3 — in 12, 2 — in 4 ( Fig. 5
).
Rice.
5. Dynamics of the frequency of exacerbations per year in group 2. Thus, the average number of exacerbations per year in the group of patients who received Alflutop intramuscularly was 4.9 cases in 2013, 4.7 in 2014, 4.5 in 2015, 4.3 in 2021 , in 2017 3.9; i.e., it decreased by 20% over 5 years (Fig. 6)
.
Rice. 6. Dynamics of the average number of exacerbations per year.
Which is better: Dona or Alflutop?
The doctor decides which drug will be more effective, Dona or Alflutop, based on tests. This takes into account the patient’s age, tendency to allergic manifestations, and individual characteristics (fear of pain).
For osteochondrosis
Experts recommend using Alflutop for this disease if there is acute pain. The medicine acts quickly: it relieves pain and stops the inflammatory process. If the disease has already become chronic and causes dull, aching pain, it is better to start taking Don. This medication can be used for a longer time than its analogue. Long-term use of the latter guarantees a more complete restoration of connective tissue, which means the rate of negative changes will be reduced.
In injections
Alflutop in this dosage form is superior to its analogue in the speed of onset of the therapeutic effect. Immediately after the start of treatment, mobility returns to the joint and pain goes away.
Comparison of addiction between Dona and Alflutop
Like safety, addiction also involves many factors that must be considered when evaluating a drug.
So, the totality of the values of such parameters as “o syndrome” in Dona is quite similar to the similar values in Alflutop. Withdrawal syndrome is a pathological condition that occurs after the cessation of intake of addictive or dependent substances into the body. And resistance is understood as initial immunity to a drug; in this it differs from addiction, when immunity to a drug develops over a certain period of time. The presence of resistance can only be stated if an attempt has been made to increase the dose of the drug to the maximum possible. At the same time, Dona has quite a small amount of “syndrome”, however, the same as Alflutop.
Can I take it together?
Despite the compatibility, it makes no sense to take both drugs at the same time, because the active ingredient is the same. You cannot resort to a combination of drugs in the hope of an accelerated result.
Taking medications together may cause excessive accumulation of glucosamines in the body and unexpected side effects.
This can lead to serious consequences - functional and structural disorders in the digestive and excretory systems. Elimination of such pathologies will require long-term drug treatment and may require a hospital stay.
Reviews from doctors about Don and Alflutop
Pavel, orthopedic surgeon, Anapa: “Both drugs have proven their effectiveness. I prescribe them to my patients diagnosed with spondylosis, coxarthrosis, osteochondrosis and other diseases of the musculoskeletal system, accompanied by degenerative changes. They give lasting results, but preventive treatment must be carried out at least 2 times a year. Alflutop contains a highly active biological concentrate that eliminates pain and stops inflammation. Dona is able to stop the destructive processes in creatures.”
Sergey, rheumatologist, Severodvinsk: “Both medications have a positive effect on the condition of the ligamentous apparatus, restore mobility to the joints and increase the range of motion. The use of both Dona and Alflutop helps to avoid muscle atrophy, which occurs due to negative changes in the joint. The use of drugs consolidates the effect of NSAIDs. Both drugs improve joint nutrition, stimulate regeneration processes, causing rapid restoration of cartilage tissue. Relapses of the disease with regular use of both drugs are rare.”
Material and methods
The case histories of 87 patients with spondyloarthrosis in the structure of occupational pathology of the musculoskeletal system from physical overload and functional overstrain were retrospectively assessed. All patients underwent clinical and physiological examination. Duration of observation: 5 years (2013–2017).
Inclusion criteria: age 18 years and older, presence of facet syndrome at the lumbosacral and/or cervical level, confirmed by neuroimaging, and signed informed consent.
Exclusion criteria: contraindications in accordance with the instructions for use of the drug Alflutop, taking chondroprotectors for 1 month before inclusion in the study. During hospital observation, patients did not take NSAIDs or analgesics. Additionally, B vitamins, muscle relaxants (tizanidine or tolperisone), metabolites were prescribed, physiotherapy was carried out according to indications (sinusoidal modulated current therapy, diadynamic therapy, electrophoresis of drugs, for example halidor), massage.
All observation cases were divided into two groups: group 1 consisted of 31 patients (20 women, 11 men), group 2 - 56 patients (25 women, 31 men). In group 1, Alflutop was injected 10 times paravertebrally into four points at the lumbosacral level (2 ml) or into two points at the lumbosacral level (1 ml) and two points at the cervical level (1 ml). In group 2, Alflutop was administered 10 times intramuscularly, 1-2 ml deep.
Demographic information is shown in the table.
Group Demographics
Of the peculiarities in the groups, it is worth noting that among the patients of group 1, 3 women and 5 men suffered from vibration disease, the remaining 23 patients suffered from muscular-tonic and radicular compression syndromes of the cervical and/or lumbosacral level. In all patients, neuroimaging reveals spinal spondyloarthrosis.
In group 2, 8 patients were diagnosed with vibration disease with radiculopathy of the lumbosacral level, the remaining 48 patients had diagnosed muscular-tonic and radicular compression syndromes of the cervical and/or lumbosacral level. Neuroimaging revealed spinal spondyloarthrosis in all patients.
All patients underwent a standard neurological examination. The main tool for pain assessment was the visual analogue scale (VAS). The number of exacerbations of back pain over 1 year was also assessed.
For statistical analysis, the R software package was used (The R Project for Statistical Computing, https://www.r-project.org). All statistical tests were two-sided, with a significance level of 5%; all confidence intervals (CIs) were two-sided 95%; Continuous data are represented by the number of observations, mean with standard deviation (SD), median (Me) and range; discrete quantities are described by absolute and relative frequencies.
Patient reviews
Oksana, 49 years old, Serpukhov: “Chondroprotectors are not cheap, but surgical intervention causes more harm to the body. Alflutop helped avoid surgery when diagnosed with coxarthrosis of the hip joint. Thanks to the medicine, the continuous pain went away (both when walking and at rest). The drug is safe for disorders of the digestive system. I noticed that the medicine manifests its pain-relieving properties even faster when the course is repeated, and the effect lasts longer.”
Vyacheslav, 28 years old, Moscow: “A sports career is associated with injuries. On the recommendation of an orthopedist, I purchased Don’s product. It helps restore injured joints well and reduces the time to return to good physical shape. Regular preventive courses with the drug help maintain the motor capabilities of the joints. Exacerbations are now rare, and this makes it possible not to miss training.”