Diagnosis and treatment of gouty arthritis
Gout is a chronic progressive disease associated with a disorder of purine metabolism, which is characterized by an increase in uric acid in the blood and deposition of sodium salt of uric acid (urate) in the tissues of the musculoskeletal system and internal organs with the development of recurrent acute arthritis and the formation of gouty nodules (tophi).
Gout is one of the “old” diseases and has been known since ancient times. The term gout comes from the Greek words pus, meaning foot, and agra, meaning grip. Thus, already in the name of the disease one of the cardinal manifestations of gouty arthritis is emphasized. Gout is considered not only as an ailment in which the pathological process is localized in the musculoskeletal system, but also as a systemic disease characterized by damage to vital organs, especially the kidneys. The prevalence of gout in different regions varies widely and is largely related to the nutritional habits of the population, averaging 0.1%. In the US, this figure is 0.84% (this figure may be overestimated).
Gout affects predominantly men (male/female ratio is 9:1). Men normally have higher levels of uric acid. In women of reproductive age, increased estrogen content increases the renal clearance of urate. In the postmenopausal period, their uric acid levels are the same as in men of the same age. Therefore, if the peak incidence in men falls at the age of 35–50 years, then in women it falls at 55–70 years. However, gout can develop at a younger age and is observed even in children.
As is known, uric acid is the end product of the breakdown of purines and is excreted from the body by the kidneys. In healthy individuals, 400–600 mg of uric acid is excreted in the urine in 24 hours. To understand the pathogenesis of gout, one should focus on the clearance of uric acid. It characterizes the volume of blood that can be cleared of uric acid in the kidneys in 1 minute. Normally, this figure is 9 ml/min. The source of uric acid formation in the body is purine compounds, which come from food or are formed in the body during nucleotide metabolism. In blood plasma, uric acid is found in the form of free sodium urate. Normally, the upper limit of this indicator for men is 0.42 mmol/l (7 mg%) and for women - 0.36 mmol/l (6 mg%). Uric acid levels above these numbers are regarded as hyperuricemia and are considered a high risk factor for the development of gout. Thus, according to the Framingham study, the development of gouty arthritis is observed in 17% of men and women with uricemia 7.0–7.9 mg%, in 25% with 8–8.9 mg% and in 90% with uric acid levels above 9.0 mg%.
If there is a persistent increase in uric acid in the blood serum above the level for a given individual, it begins to be deposited in the tissues in the form of free sodium urate, which is converted into uric acid in the urinary tract.
The following clinical variants of gout are distinguished:
- asymptomatic hyperuricemia (hyperuricosuria);
- interictal gout;
- acute gouty arthritis;
- chronic tophi gout.
Hyperuricemia can occur for a long time without any subjective or objective symptoms and is only accidentally diagnosed during examination of the patient. However, it is not as harmless as it may seem at first glance, and is often associated with disorders of fat and carbohydrate metabolism, and, even more seriously, leads to urate nephropathy. It should be noted that the definition of “asymptomatic gout” is conventional. To identify it, it is necessary to re-examine the level of uric acid, especially in the “gouty personality,” that is, in young men with an addiction to alcohol, obesity and arterial hypertension. In some cases, the period of asymptomatic (chemical) hyperuricemia lasts several years and only after this does the clinical presentation of gout occur. It should be borne in mind that hyperuricemia is usually preceded by hyperuricosuria. Therefore, in patients with uric acid diathesis, it is necessary to re-examine the level of uric acid not only in the blood, but also in the urine in order to timely detect gout.
The level of uric acid in the blood can increase under the influence of various factors, both internal and external. These factors contribute to either an increase in the formation of endogenous purines or a slowdown in their excretion by the kidneys. From this perspective, there are two types of hyperuricemia - metabolic and renal. The metabolic type is characterized by an increase in the synthesis of endogenous purines in the presence of high uricosuria and normal clearance of uric acid. In contrast, in the renal type there is low clearance of uric acid and, consequently, impaired renal excretion of uric acid. The types of hyperuricemia presented are of paramount importance in the selection of anti-gout disease-modifying drugs used in the treatment of this disease.
Reasons for increased purine biosynthesis
Hereditary factors:
- decreased activity of hypoxanthine-guanine phosphoribosyltransferase;
- high phosphoribosyltransferase activity;
- deficiency of glucose-6-phosphate.
Nosological forms and clinical syndromes:
- increased nucleotide metabolism (polycythemia vera and secondary erythrocytosis, acute and chronic leukemia, lymphoma, hemolytic anemia, hemoglobinopathies, pernicious anemia, etc.);
- tumors;
- psoriasis and psoriatic arthritis;
- systemic lupus erythematosus, systemic scleroderma;
- hyperparathyroidism;
- obesity;
- Gaucher disease;
- Infectious mononucleosis;
- tissue hypoxia.
Medicines, diet and chronic intoxication:
- ethanol;
- a diet high in purines;
- fructose;
- a nicotinic acid;
- cytotoxic drugs;
- warfarin;
- ethylamine-1,3,4-thiadiazole.
Reasons for slower excretion of uric acid by the kidneys
Nosological forms and clinical syndromes:
- chronic renal failure;
- kidney diseases with predominantly interstitial and tubular disorders (polycystic kidney disease, analgesic nephropathy, hydronephrosis);
- lead nephropathy;
- dehydration;
- diabetic ketoacidosis;
- hyperproduction of lactic acid;
- preeclampsia;
- obesity;
- hyperparathyroidism;
- hypothyroidism;
- sarcoidosis;
- chronic beryllium intoxication.
Medicines and chronic intoxications:
- thiazide diuretics;
- cyclosporine;
- low doses of salicylates;
- anti-tuberculosis drugs (pyrazinamide);
- ethanol;
- Levodopa.
There are also primary and secondary gout. With primary gout, there is no underlying disease that precedes its development. The basis of such gout is a family-genetic abnormality of purine metabolism, determined by several genes, or the so-called “constitutional dyspurism”. Studies of urate homeostasis have shown an autosomal dominant mode of inheritance of such anomalies. In particular, this is observed in congenital disorders in the content of enzymes that occupy a key position in purine metabolism. Thus, with a decrease in the activity of hypoxanthine-guanine phosphoribosyltransferase, an increase in the resynthesis of purines from nucleotides occurs, which contributes to the development of Lesch-Nyhan syndrome. This syndrome occurs only in children and young adults and usually results in urate nephropathy and death. With a high content of phosphoribosyl pyrophosphate, a metabolic type of hyperuricemia is also observed, since this enzyme is involved in the synthesis of uric acid precursors. As for secondary gout, it is one of the syndromes of another disease, a “second disease” that develops in many pathological processes and most often in chronic renal failure.
The clinical picture of acute gouty arthritis is of great importance in recognizing gout, especially its early stages. It is well known, but the frequency of diagnostic errors in the first year of the disease reaches 90%, and after 5–7 years the correct diagnosis is made only in half of the cases. Late diagnosis is associated with underestimation of the classic early signs of the disease, as well as with the diversity of the onset and course of gout. Its diagnosis is based on the characteristics of the clinical picture of the disease, increased levels of uric acid in the blood and the detection of sodium urate crystals in tissues. In practice, the following so-called Rome diagnostic criteria for gout are widely used:
- acute attack of arthritis affecting the metatarsophalangeal joint of the big toe;
- gouty nodes (tophi);
- hyperuricemia (the level of uric acid in the blood serum is higher than the physiological norm);
- detection of urate crystals in synovial fluid or tissues.
The diagnosis of gout is considered reliable if any two of the four criteria are met.
Less common are the diagnostic criteria for gout proposed by the American College of Rheumatology (ACR) in 1977, which characterize acute inflammatory arthritis or its recurrent attacks rather than gout in general. According to these criteria, a reliable diagnosis is made if 6 out of 12 signs are present:
- more than one attack of acute arthritis;
- development of the most acute inflammatory process during the first day;
- monoarthritis;
- redness of the skin over the affected joint;
- pain or swelling of the first metatarsophalangeal joint;
- asymmetrical lesion of the first metatarsophalangeal joint;
- asymmetrical damage to the tarsal joints;
- the presence of formations resembling tophi;
- asymmetric swelling within the joint (radiological sign);
- subcortical cysts without erosions;
- hyperuricemia;
- sterile joint fluid.
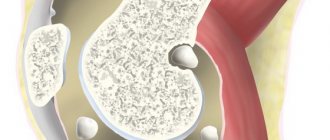
In the first 3–4 years, gout occurs as a recurrent acute inflammatory monoarthritis with complete regression and restoration of joint function, while the interictal period lasts from several months to 1–2 years. Subsequently, this period is shortened, more and more new joints are involved in the process, and inflammatory phenomena are localized not only in the joints of the feet, but spread to the joints of the upper extremities, which usually coincides with the formation of tophi. Tophi are deposits of uric acid crystals. They appear on average 6 years after the first attack of gout, but sometimes after 2-3 years. Urates are most often deposited on the surface of the articular cartilage, in the synovial membrane, synovial sheaths, tendons, and also in the subcortical region of the epiphyses of bones. Most often they are located on the ears and on the back of the elbow joints. Tophi are divided into single and multiple, and are also classified according to their size, with small tophi being up to 1 cm in diameter, medium - from 1 to 2.5 cm and large - more than 2.5 cm. Gouty nodes with localization in the musculoskeletal system are the main element in the formation of chronic gouty arthritis. Tophi can be located in the kidneys and other visceral organs.
The chronic course of gout is not limited only to the involvement of joints and the formation of tophi, but is also characterized by damage to internal organs. Gouty nephropathy is the most important manifestation of gout from a prognostic point of view and the most common cause of death in this disease. Some variants of gouty nephropathy include acute uric acid blockade of the renal tubules, uric acid nephrolithiasis caused by the deposition of uric acid salts in the calyces and pelvis of the kidneys, chronic urate nephropathy and diffuse glomerulonephritis. Acute uric acid blockade of renal tubules occurs, for example, when a tumor disintegrates as a result of massive drug or radiotherapy. Chronic urate nephropathy is associated with the deposition of urate in the interstitium of the kidneys, and the development of diffuse glomerulonephritis is associated with immune disorders in individuals with dysregulation of purine metabolism. Such glomerulonephritis, in its immunomorphology, is most often mesangioproliferative and with it deposits of IgG and complement are detected. It should be borne in mind that gout is often associated with such pathological conditions as arterial hypertension, obesity, hyperlipidemia, fatty liver, atherosclerosis, cerebrovascular accidents, and alcohol dependence.
The course of gout is characterized by a variety of rates of disease development. A relatively benign course with rare attacks, slight hyperuricemia and uricosuria and long-term persistence of functional insufficiency of the musculoskeletal system is possible. In other cases, on the contrary, from the very beginning of the disease there are frequent attacks of acute arthritis with severe pain or continuous attacks with multiple joint damage over several weeks or months (gouty status). Gout refractory to therapy leads to the rapid development of functional failure of the joints and kidneys.
The basis for identifying variants of the course of gout are: the number of attacks of arthritis during the year, the number of affected joints, the severity of osteochondral destruction, the presence of tophi, kidney pathology and its nature.
Variants of the course of gout
Mild: attacks of arthritis 1–2 times a year and involve no more than 2 joints, there is no kidney damage or destruction of joints, there are no tophi or they are single and do not exceed 1 cm in diameter.
Moderate severity: 3–5 attacks per year, damage to 2–4 joints, moderately severe osteoarticular destruction, multiple small tophi, kidney damage is limited to nephrolithiasis.
Severe: frequency of attacks more than 5 per year, multiple joint damage, multiple large tophi, severe nephropathy.
The main goals of therapy for gouty arthritis are:
- relief of acute attacks of the disease;
- reduction of urate content in the body;
- treatment of chronic polyarthritis;
- impact on extra-articular pathology.
Relief of acute gouty arthritis is carried out with anti-inflammatory drugs. For these purposes the following are used: colchicine - colchicine, colchicum-disperse; non-steroidal anti-inflammatory drugs (NSAIDs) - Voltaren, Diclovit, Dicloran, Celebrex, Movalis); corticosteroids - polcortolone, prednisolone, methylprednisolone; or a combination of NSAIDs and corticosteroids - Ambien. Both colchicine and NSAIDs reverse acute arthritis within hours, whereas in untreated patients it can last several weeks. Until recently, it was believed that the best drug for relieving an attack of acute arthritis due to gout was colchicine. The pronounced and rapid (within 48 hours) effect of colchicine was considered as one of the diagnostic signs of this disease. Colchicine can prevent further development of an acute attack of gout when administered in the first 30–60 minutes of an attack. Its therapeutic effect is due to the inhibition of the phagocytic activity of neutrophils. In an acute attack of gout, monosodium urate salts, phagocytosed by neutrophils, due to their membranolytic action, lead to the death of these cells and the release of lysosomal enzymes, which are responsible for the development of acute inflammation.
Among NSAIDs, preference is given to indomethacin (Indotard, Methindol) and diclofenac (Voltaren, Dicloran, Diclofen). These drugs are prescribed at a dose of 200–250 mg/day, with most of the daily dose used in the first hours of an attack. Controlled studies have not revealed a higher effectiveness of traditional NSAIDs compared to selective COX-2 inhibitors (nimesil, nimulid, Celebrex), such as celecoxibs (coxib, celebrex). However, a final judgment on the comparative effectiveness of these drugs can only be made through further research. The daily dose of colchicine is 4–6 mg/day, with the patient taking 2/3 of this dose before 12 noon on the first day of the attack. Typically, a single dose is 0.6 mg and is taken every hour until there is a clear reduction in gouty inflammation. After a significant reduction in inflammation, the dose of colchicine begins to be reduced by 0.6 mg 2 times a day, until complete withdrawal. Often, patients are unable to increase the daily dose to the optimal dose due to the occurrence of adverse reactions. The main side effects of colchicine are nausea, vomiting, diarrhea, hemorrhagic gastroenteritis, leukopenia, and neuropathy are also possible. In case of gouty status, characterized by continuous attacks of acute arthritis, refractory to NSAID therapy, intravenous administration of colchicine is possible.
Anti-gout therapy (basic, disease-modifying) is aimed at preventing relapses of acute arthritis, reducing the level of uric acid in the blood, preventing further formation of tophi and their reverse development. All anti-gout drugs are divided into two large groups: uricodepressors (uricostatics) and uricosurics. Uricodepressors inhibit the synthesis of uric acid by inhibiting the enzyme xanthine oxidase, which converts hypoxanthine into xanthine and xanthine into uric acid. Uricosurics increase the excretion of uric acid by inhibiting the reabsorption of urate by the renal tubules.
The drugs of the first group include allopurinol (allopurinol, allopurinol-egis, allupol, purinol, remid, thiopurinol, milurite), which occupies a leading position among other anti-gout drugs. Indications for the use of allopurinol are metabolic gout, high hyperuricemia, frequent acute attacks of arthritis, uric acid disease, genetically determined deficiency of hypoxanthine-guanine phosphoribosyltransferase. The use of allopurinol is also possible in patients with gouty nephropathy with initial manifestations of chronic renal failure and slight azotemia. The initial dose of allopurinol is 300 mg/day. If this dose is ineffective, it is increased to 400–600 mg/day, and when a clinical effect is achieved, it is gradually reduced. The maintenance dose is determined by the level of hyperuricemia and is usually 100–300 mg/day.
Allopurinol promotes the disappearance of acute arthritis attacks or their noticeable weakening, the reverse development of tophi and their distinct softening, the reduction of uric acid levels to subnormal levels, the normalization of urinary syndrome indicators, the reduction of renal colic and renal excretory function. In some patients, it initially causes an increase in uric acid levels and an exacerbation of gouty arthritis, so in the first stage of therapy it is combined with anti-inflammatory drugs, in particular low doses of colchicine or NSAIDs. For this reason, it should not be taken for acute gouty arthritis. When treating with allopurinol, adverse reactions often develop, which are manifested by gastrointestinal toxicity, allergic reactions (skin rash, eosinophilia), hepatotoxicity with an increase in serum aminotransferases.
Uricosuric drugs, which are weak organic acids, are less important in the treatment of gout than uricostatics. They should not be prescribed for high levels of uric acid in the blood, as well as for nephropathy, even with initial manifestations of renal failure. Among the uricosuric drugs, sulfinpyrazone and probenecid are especially widely used in the United States. Sulfinpyrazone (sulfinpyrazone, apo-sulfinpyrazone, anturan) is prescribed at 200–400 mg/day in two doses. It, like other uricosuric drugs, is taken with a large amount of fluid, which should be alkalized to prevent nephrolithiasis. Adverse reactions are relatively common and include gastric and intestinal dyspepsia, leukopenia, and allergic reactions. Contraindications to the use of sulfinpyrazone are gastric ulcers and, of course, gouty nephropathy.
Probenecid (benemid) is a derivative of benzoic acid. The drug is prescribed at 1.5–2.0 g/day. Benzoic acid is found in cranberries, as well as lingonberry berries and leaves. Therefore, decoctions and fruit drinks from the berries and leaves of these plants are indicated for patients with gout and, to a greater extent, for patients with gouty nephropathy, especially since in addition to benzoic acid they contain hippuric acid, which has uricoseptic properties. Uricosuric activity is inherent in the angiotensin II receptor blocker and fenofibrate (grofibrate, nofibal). The most effective are benzbromarone derivatives, which have not only uricosuric properties, but also uricodepressor properties. They are used as monotherapy or in combination with allopurinol. Such a combination drug is allomaron. Allomaron contains 20 mg benzbromarone and 100 mg allopurinol, and is usually taken 1 tablet 2 times a day.
An integral part of complex therapy for gout are alkalizing drugs and alkalizing solutions, which can reduce the risk of developing nephropathy and, in particular, urolithiasis. These drugs include Magurlit, Blemaren and Uralit. Their use should be regularly monitored by urine pH. In addition to these remedies, you can take baking soda 2-4 g per day or alkaline mineral water.
If the symptoms of arthritis are pronounced, it is also necessary to carry out local treatment (dolobene, finalgon, dicloran plus, doltit cream, nemulide gel, bischofite gel).
Diet for gout is given the greatest importance compared to other rheumatic diseases. It involves reducing the total caloric intake of food, especially since with gout, increased body weight is usually observed. It is necessary to reduce the intake of exogenous purines and animal fats into the body. Fats reduce the excretion of uric acid by the kidneys. You should be extremely careful when consuming any alcoholic beverages, including beer and red wine. Exclude liver, kidneys, fatty meats, meat broths, smoked meats, peas, beans, lentils, spinach, cauliflower, sprats, and herring from the diet. You should limit your meat consumption to 2-3 times a week, and it is better to eat it boiled.
The combination of a strict diet with long-term use of anti-gout drugs, as well as active influence on diseases that increase the level of uric acid in the blood, can significantly slow down the rate of progression of osteochondral destruction, prevent further formation of tophi and maintain the functional state of the musculoskeletal system and kidneys.
V. V. Badokin, Doctor of Medical Sciences, Professor of the Russian Medical Academy of Postgraduate Education, Moscow
Means that promote the removal of uric acid and the removal of urinary calculi (stones)
Home Medical Encyclopedia Medicines Medicines that affect kidney function
AVISAN (Avisanum)
Contains a sum of substances obtained from the fruits of Ammi Visnaga L..
Pharmachologic effect. It has antispasmodic (relieving spasms) properties. By relaxing the muscles of the ureters, it promotes the advancement and passage of ureteral stones.
Indications for use. Spasm (sharp narrowing of the lumen) of the ureters, renal colic.
Method of administration and dose. Orally 0.05-0.1 g 3-4 times a day after meals for 1-3 weeks.
To facilitate the removal of stones from the urinary tract, it is recommended that the patient be given a large amount of fluid while taking Avisan. In the absence of contraindications from the cardiovascular system and kidneys, the patient drinks 1.5-2 liters of water or tea within 2-3 hours. This technique is repeated after a few days. The patient should be under the supervision of a doctor.
Side effect. In some cases, dyspepsia (digestive disorders).
Release form. Film-coated tablets, 0.05 g in a package of 25 pieces.
Storage conditions. In a dry place, protected from light.
ALLOPURINOL (AUopurinolum)
Synonyms: Milurit, Apurin, Zilorik, Allopur, Atizuril, Foligan, Goticur, Lisurin, Petrazin, Prinol, Piral, Purinol, Uridoside, Uriprim, Xanthurate.
Pharmachologic effect. By inhibiting xanthine oxidase, it inhibits the synthesis of uric acid, which helps reduce the urate content in the serum and prevents its deposition in the kidneys.
Indications for use. For the treatment and prevention of diseases accompanied by hyperuricemia (increased levels of uric acid in the blood) and the formation of urate stones (consisting of uric acid salts): primary and secondary gout, urolithiasis with the formation of calculi (stones) containing urate, primary and secondary hyperuricemia.
Method of administration and dose. Orally after meals, 0.1 g 3-4 times a day, the daily dose can be increased to 0.8 g (in four divided doses).
In severe cases of gout, with significant deposits of urate in tissues and high hyperuricemia (over 7 mg%), up to 0.6-0.8 g is prescribed in fractional doses (no more than 0.2 g per dose) for 2-4 weeks, then switch to maintenance doses (0.1-0.3 g per day), which are given long-term (for several months).
When allopurinol is stopped, uricemia (increased uric acid in the blood) and uricosuria (increased excretion of uric acid in the urine) return to baseline levels, so treatment must be long-term. Gaps in taking the drug for more than 2-3 days are undesirable.
When treating with allopurinol, it is necessary to maintain diuresis (urination) at a level of at least 2 liters per day; It is desirable that the urine reaction be neutral.
Side effect. At the beginning of treatment, an exacerbation of the disease, sometimes dyspeptic symptoms (digestive disorders), eosinophilia (increased number of eosinophils in the blood), skin rashes, fever (sharp increase in body temperature).
Contraindications. Renal failure with impaired excretory function, pregnancy.
Release form. Tablets of 0.1 g in a package of 50 pieces.
Storage conditions. List B. In a place protected from light.
BENZOBROMARON (Benzobromarone)
Synonyms: Normurat, Khipurik, Dezurik, Azabromaron, Exurat, Maksurik, Minurik, Urikonorm, Urikozurik, Urinorm.
Pharmachologic effect. Benzobromarone has a strong uricosuric effect (increases the excretion of uric acid). The effect is mainly due to inhibition of the absorption of uric acid in the proximal (located in the central part of the kidney) renal tubules and an increase in the excretion of uric acid by the kidneys. In addition, the drug inhibits (suppresses the activity) of enzymes involved in the synthesis of purines. Under the influence of benzobromarone, the excretion of uric acid through the intestines is enhanced.
Indications for use. Used for hyperuricemia (increased levels of uric acid in the blood) (for arthritis / joint inflammation / with hyperuricemia, hematological diseases, psoriasis, etc.) and gout.
Method of administration and dose. Prescribed to adults orally during meals, starting with 0.05 g (50 mg = '/2 tablets) 1 time per day, and if the urate level in the blood is not sufficiently reduced - 1 tablet per day.
For acute attacks of gout, it is sometimes prescribed in short courses of 1/2 tablet 3 times a day for 3 days.
During treatment, to prevent the deposition of calculi (stones) in the urinary tract, the patient should drink at least 2 liters of fluid per day.
Side effect. The drug is usually well tolerated. In some cases, gastrointestinal disorders (diarrhea) and allergic skin reactions are possible. With gout, joint pain may intensify in the first days; in these cases, non-steroidal anti-inflammatory drugs are prescribed.
Contraindications. Pregnancy, breastfeeding, severe liver and kidney damage. The drug should not be prescribed to children.
Release form. Tablets of 0.1 g in a package of 30 pieces and tablets containing 0.08 g (80 mg) of micronized benzobromarone (hipuric).
Storage conditions. In a place protected from light.
Blemaren
Synonyms: K-Na hydrogen citrate, Soluran.
Pharmachologic effect. Helps neutralize urine, allows you to maintain pH (an indicator of the acid-base state) within 6.6-6.8, which creates optimal conditions for increasing the dissolution of uric acid. Long-term use of the drug leads to the dissolution of uric acid stones and prevents their appearance.
Indications for use. Urolithiasis with a predominance of urates, prevention of the formation of uric acid stones.
Method of administration and dose. Doses are set individually, on average 3-6 g (1-2 dosed spoons) 2-3 times a day after meals. The drug is diluted in water or fruit juice. Treatment is carried out under the control of urine pH 3 times a day using the indicator included with the drug.
Alkaline urine (pH above 7.0) should be avoided, as this promotes the formation of phosphates.
Side effect. Rarely - disorders of the gastrointestinal tract.
Contraindications. Chronic urinary tract infection with urea-splitting bacteria
acute and chronic renal failure, circulatory failure.
Release form. In a package of 300 g with a dosed spoon, a control calendar and an indicator for determining pH. Composition per 100 g of granules: anhydrous citric acid - 39.90 g, potassium bicarbonate - 32.25 g, anhydrous sodium citrate - 27.85 g.
Storage conditions. In a well-closed container in a dry place.
Knotweed (Herba Polygon! avicularis)
Contains flavonol glycosides - quercetin, hyperoside, avicularin; tannins.
Pharmachologic effect. Anti-inflammatory and promoting the removal of calculi (stones).
Indications for use. As an anti-inflammatory agent that promotes the passage of stones for stones in the kidneys and bladder.
Method of administration and dose. Used as an infusion (10.0:20.0-15.0:20.0) 2 tablespoons 3 times a day before meals.
During treatment, to prevent the deposition of stones in the urinary tract, the patient should drink at least 2 liters of fluid per day.
Release form. Packaged in 100 g.
Storage conditions. In a dry, cool place.
MADDER EXTRACT DRY (Extractum Rubiae tinctorum siccum)
Extract from rhizomes and roots of perennial herbaceous plants, madder (Rubia tinctorum L.) and Georgian madder (Rubia iberica Fisch.), fam. madder (Rubicea). Contain at least 3% anthracene derivatives.
Pharmachologic effect. It has an antispasmodic (relieving spasms) and diuretic effect; promotes loosening of urinary stones containing calcium and magnesium phosphates.
Indications for use. Urolithiasis disease.
Method of administration and dose. Orally 0.25-0.75 g 2-3 times a day in 1/2 glass of warm water. The course of treatment is 20-30 days.
Side effect. Colors urine reddish.
Release form. Tablets of 0.25 g in a package of 100 pieces.
Storage conditions. In a well-closed container.
TABLETS "MARELIN" (Tabulettae "Marelinum" obductae)
A combined preparation containing dry madder extract, dry horsetail herb extract, dry goldenrod extract, single-substituted magnesium phosphate, corglycon, kellin, salicylamide.
Pharmachologic effect. It has an antispasmodic (relieving spasms) and anti-inflammatory effect. Promotes the removal of kidney stones (stones), consisting of calcium oxalates and calcium phosphates. Reduces or relieves pain in renal colic. With an alkaline reaction of urine, the pH (an indicator of the acid-base state) shifts to the acidic side.
Indications for use. Urolithiasis disease.
Method of administration and dose. If stones are present, take 2-4 tablets 3 times a day orally (before meals) daily for 20-30 days. Treatment is carried out in repeated courses with an interval of 1-1.5 months.
To prevent relapse (re-deposition of stones) after surgical removal of stones or their spontaneous passage, 2 tablets are prescribed
3 times a day every day for 2-3 months. If necessary, the course of treatment is repeated after 4-6 months.
To facilitate the removal of stones from the urinary tract, increased fluid intake into the body is recommended simultaneously with taking the drug. In the absence of contraindications from the cardiovascular system and kidneys, the patient should take at least 1.5-2 liters of liquid (mineral alkaline water, tea, fruit juices).
For patients with inflammatory diseases of the gastrointestinal tract, the drug is prescribed after meals (dyspeptic symptoms /digestive disorders/ and exacerbation of peptic ulcer are possible).
Contraindications. Acute and chronic glomerulonephritis (kidney disease).
Release form. Tablets containing: dry madder extract - 0.0325 g, dry horsetail herb extract - 0.015 g, dry goldenrod extract - 0.025 g, single-substituted magnesium phosphate - 0.01 g, corglycon - 0.000125 g, kellin - 0, 0025 g, salicylamide -0.035 g, coated, in glass jars of 120 pieces.
Storage conditions. In a dry, cool place, protected from light.
Cystenal
Pharmachologic effect. Antispasmodic (relieving spasms), anti-inflammatory and diuretic (diuretic) agent.
Indications for use. Urolithiasis disease.
Method of administration and dose. Orally, half an hour before meals, 3-4 drops (for an attack, 20 drops) on a piece of sugar; for frequent attacks, 10 drops 3 times a day.
To facilitate the removal of stones from the urinary tract, increased fluid intake into the body is recommended simultaneously with taking the drug. In the absence of contraindications from the cardiovascular system and kidneys, the patient should take at least 1.5-2 liters of liquid (mineral alkaline water, tea, fruit juices).
Contraindications. Glomerulonephritis (kidney disease), severe renal dysfunction, gastric ulcer.
Release form. In bottles of 10 ml. Ingredients: madder root tincture - 0.093 g, magnesium salicylate - 0.14 g, essential oils - 5.75 g, ethyl alcohol - 0.8 g, olive oil up to 10 ml.
Storage conditions. Under normal conditions.
MAGURLIT (Magurlit)
Pharmachologic effect. The drug is designed to shift the pH (an indicator of the acid-base state) of urine towards an alkaline reaction, as well as to inhibit the formation and dissolution of stones consisting of calcium oxalate, as well as a mixture of uric acid with calcium oxalate.
Indications for use. Magurlit is used to dissolve and prevent the re-formation of urinary stones in cases with persistent acidity of urine (pH less than 5.5).
Method of administration and dose. Taken orally. The average dose for adults is 6-8 g per day (2 g early in the morning, 2 g in the afternoon and 2 or 4 g late in the evening). Take with water (or fruit juice).
To further clarify the dose, the urine pH is determined daily using the indicator paper included with the drug, comparing the color with the attached color scale; The pH of fresh urine, determined in the morning, afternoon and evening before taking the drug, should, with the correct dosage, be in the range from 6.0 to 6.7-7.0. Exceeding this value is necessary
avoid, since alkaline urine (pH above 7.0) can form phosphate stones. To maintain the pH at the specified level, the dose of the drug must be selected individually.
To facilitate the removal of stones from the urinary tract, increased fluid intake into the body is recommended simultaneously with taking Magurlit. In the absence of contraindications from the cardiovascular system and kidneys, the patient should take at least 1.5-2 liters of liquid (mineral alkaline water, tea, fruit juices).
The drug can be used for a long time - continuously or intermittently.
Treatment should be carried out under medical supervision.
Side effect. During treatment, disturbances in the activity of the gastrointestinal tract may be observed, usually resolving without stopping the course of treatment.
Contraindications. Chronic urinary tract infections, circulatory failure (due to the large amount of sodium and potassium in the drug).
Release form. In bags of 2 g of the drug in a package of 100 pieces with the attachment of indicator papers, a color scale and tweezers.
Storage conditions. In a well-packed container.
OLIMETHIN (Olimetinum)
Synonyms: Enatin, Rowatin, Rovahol.
Pharmachologic effect. It has a diuretic, anti-inflammatory, antispasmodic (relieving spasms), choleretic effect.
Indications for use. Kidney stones and cholelithiasis.
Method of administration and dose. Orally before meals, 2 capsules 3-5 times a day. For the purpose of prevention (after stones pass), 1 capsule per day for a long time.
Contraindications. Urinary disorders, acute and chronic glomerulonephritis (kidney disease), hepatitis, gastric ulcer.
Release form. Capsules of 0.5 g in a package of 12 pieces. Composition of one capsule: peppermint oil - 0.0085 g, purified turpentine oil - 0.01705 g, essential calamus oil - 0.0125 g, olive oil - 0.46025 g, purified sulfur -0.0017 g.
Storage conditions. In a dry, cool place, protected from light.
Pinabinum
50% solution of the heavy fraction of essential oils (from pine or spruce needles) in peach oil.
Pharmachologic effect. It has an antispasmodic (relieving spasms) effect on the muscles of the urinary tract, and a bacteriostatic (preventing the proliferation of bacteria) effect.
Indications for use. Kidney stone disease, renal colic.
Method of administration and dose. Orally, 5 drops 3 times a day with sugar 15-20 minutes before meals. The course of treatment is 4-5 weeks; for renal colic, up to 20 drops of sugar once.
Side effect. Large doses of the drug can cause irritation of the mucous membrane of the stomach and intestines, hypotension (lowering blood pressure).
Contraindications. Nephritis, nephrosis (kidney diseases).
Release form. In bottles of 25 ml of a 50% solution in peach oil.
Storage conditions. List B. In a cool place.
SULFIN PYRAZONE (Sulfmpyrazone)
Synonyms: Anturan, Anturanil, Anturidin, Enturan, Pirocard, Sulfazon, Sulfison, etc.
Pharmachologic effect. It is an active uricosuric (uric acid-removing) agent.
Indications for use. Used to treat gout. Increases the excretion of uric acid through the kidneys, especially in the first stage of treatment.
Method of administration and dose. It is usually prescribed orally in a daily dose of 0.3-0.4 g (in 2-4 doses). Take after meals; It is advisable to drink it with milk.
It should be taken into account that small doses of salicylates weaken the uricosuric effect of sulfinpyrazone. The drug enhances the effect of oral anticoagulants, oral antidiabetic agents, sulfonamides, and penicillin.
Side effect. Sulfinpyrazone is usually well tolerated, but exacerbation of gastric and duodenal ulcers is possible.
At the beginning of treatment for gout, attacks may become more frequent. When “prescribing a course of treatment, first of all it is necessary to introduce a sufficient amount of fluid into the body and acidify the urine (taking sodium bicarbonate); With an acidic urine reaction, calculi (stones) may fall out in the urinary tract.
Contraindications. Peptic ulcer of the stomach and duodenum, hypersensitivity to butadiene and related drugs, severe damage to the liver and kidneys.
Release form. Tablets 0.1 g.
Storage conditions. In a place protected from light.
URODAN (Urodanum)
Pharmachologic effect. The lithium salts and piperazine included in the drug in combination with uric acid form easily soluble compounds and promote its removal from the body.
Indications for use. Gout, urolithiasis, chronic polyarthritis (inflammation of several joints).
Method of administration and dose. Orally before meals, a teaspoon in /2 glasses of water 3-4 times a day. Use for a long time (30-40 days). If necessary, the course is repeated.
Release form. Granules 100 g. Composition: piperazine - 2.5 g, hexamethylenetetramine - 8 g, sodium benzoate - 2.5 g, lithium benzoate - 2 g, disodium phosphate (anhydrous) - 10 g, sodium bicarbonate - 37.5 g, tartaric acid - 35.6 g, sugar - 1.9 g.
Storage conditions. Regular.
UROLESAN (Urolesanum)
Pharmachologic effect. Combined herbal preparation. It has antiseptic (disinfecting) properties, increases diuresis (urination), acidifies urine, increases the secretion of urea and chlorides, enhances bile formation and bile secretion, improves hepatic blood flow.
Indications for use. Various forms of urolithiasis and cholelithiasis, salt diathesis, acute and chronic pyelonephritis (kidney disease) and cholecystitis (inflammation of the gallbladder), cholangiohepatitis (combined inflammation of the liver and bile
ducts) and dyskinesia (impaired mobility) of the biliary tract.
Method of administration and dose. 8-10 drops per lump of sugar under the tongue 3 times a day before meals. The course of treatment depends on the severity of the disease and lasts from 5 days to 1 month. For renal and hepatic colic, a single dose can be increased to 15-20 drops.
Side effect. Slight dizziness and nausea are possible. In this case, plenty of hot drinks and rest are prescribed.
Release form. Orange glass dropper bottles, 15 ml. Composition per 100 g: fir oil - 8 g, peppermint oil - 2 g, castor oil - 11 g, alcohol extract of wild carrot seeds - 23 g, alcohol extract of hop cones - 32.995 g, alcohol extract of oregano herb - 23 g , trilona - B 0.005 g.
Storage conditions. In a place protected from light at a temperature not exceeding +20 °C.
PHYTOLYSIN (Phytolysinum)
A mixture of aqueous herbal extracts and essential oil containing flavone derivatives, inositol, silicates, saponins, cineol, borneol, terpineol, camphene, etc. Contains plant extracts: parsley root, wheatgrass rhizome, horsetail grass, birch leaves, knotweed grass, etc. ., as well as oils - mint, sage, pine, orange and vanillin.
Pharmachologic effect. It has a diuretic, anti-inflammatory, analgesic and bacteriostatic (preventing the proliferation of bacteria) effect, and also facilitates the loosening and removal of urinary calculi (stones).
Indications for use. Inflammation of the urinary tract, kidneys, renal pelvis and bladder, loosening of urinary stones and facilitating their excretion in the urine.
Method of administration and dose. A teaspoon of paste is dissolved in 1/2 cup of warm sweet water. Take 3-4 times a day after meals. Take for a long time.
Contraindications. Acute inflammatory kidney diseases, nephrosis (kidney disease), phosphate lithiasis (phosphate kidney stones).
Release form. Paste in tubes of 100 g.
Storage conditions. In a cool place.
ETAMIDE (Aethamidum)
Synonyms: Etebenecid.
Pharmachologic effect. Inhibits the reabsorption (reabsorption) of uric acid in the renal tubules, promotes its excretion in the urine and reduces its content in the blood.
Indications for use. Chronic gout, polyarthritis (inflammation of several joints) with impaired purine metabolism, urolithiasis with the formation of urates.
Method of administration and dose. Orally after meals, 0.35 g 4 times a day for 10-12 days. After a 5-7-day break, treatment is continued for 7 days. If necessary, the dose is increased as prescribed by the doctor.
Side effect. Possible dyspeptic and dysuric (digestive and urinary disorders) phenomena that go away on their own.
Release form. Tablets of 0.35 g in a package of 50 pieces.
Storage conditions. In a dry place.
| print version | This information is not a guide to self-treatment. A doctor's consultation is required. |
Pharmacotherapy of gout
Treatment of gout seems to be an exhausted topic. Over the past 25 years, not a single fundamentally new anti-gout drug has been created. However, practice shows that not all issues in the treatment of gout have been resolved. One of the important problems is timely and accurate diagnosis of the disease.
The most common are the so-called Rome diagnostic criteria for gout (1961) (see box). It is necessary to make a number of comments regarding these diagnostic criteria.
They do not take into account the kidney damage that naturally occurs with gout and, in particular, the significant fact that in 40% of patients the detection of kidney stones precedes the first articular attack. The upper limits of normal uricemia given in the Rome criteria were determined using manual methods (colorimetric and enzymatic uricase). The use of the now most common automated methods for determining uric acid has led to a recalculation of normal values - they increase by 0.4–1.0 mg% or by 24–60 µmol/l (see table).
Errors in the diagnosis of gout result from ignorance of the fact that during an acute attack, the level of uric acid in many patients (according to various sources, in 39–42%) decreases to normal levels.
The most reliable diagnostic method is the detection of urate crystals using polarization microscopy
. But one must take into account the relatively low sensitivity of this research method (69%), the dependence of the results on the experience and thoroughness of the microscopist, as well as on the number of crystals and their sizes. Crystals of monosodium urate in the synovial fluid can be found (usually outside the cells) in patients with joint damage of other etiologies with concurrent asymptomatic hyperuricemia, for example, in psoriatic arthritis, hyperparathyroidism, sarcoidosis, malignant tumors, renal failure.
The striking effect of colchicine, previously considered a diagnostic sign of gout, is now not considered as such, as it can be observed in pseudogout and a number of other acute arthritis.
Methods for relieving acute gouty arthritis
There are two classic approaches to relieving a gout attack: colchicine or nonsteroidal anti-inflammatory drugs (NSAIDs)
.
It is now recognized that the overall effectiveness of these two methods is the same.
The differences are only in the speed of onset of the effect and tolerability. Colchicine begins to act faster: between 12 and 48 hours (NSAIDs - between 24 and 48 hours), but undoubtedly causes side effects more often. In the only double-blind, placebo-controlled study, colchicine
proved effective in 2/3 of patients with acute gout (placebo – in 1/3 of patients); treatment was more successful if it was started within the first 24 hours after the onset of the attack. More than 80% of patients experienced nausea, vomiting, diarrhea, or abdominal pain before complete resolution of arthritis (MJ Ahbern et al.). The standard method of using colchicine for an acute attack of gout is to administer 0.5 mg of the drug every hour. Treatment is carried out until the onset of effect, the development of side effects, or the maximum dose is reached (usually no more than 6 mg over 12 hours; in patients with renal failure and the elderly, the dose should be lower).
Among NSAIDs, preference is given to the most effective in anti-inflammatory terms: previously, as a rule, phenylbutazone was prescribed (now it is almost never used due to the risk of hematological complications), currently diclofenac sodium
or
indomethacin
(in doses up to 200 mg per day). There is a known method of simultaneous use of colchicine (in low doses of 1–1.5 mg per day) and NSAIDs.
Judging by survey data from American and Canadian doctors, the vast majority of them prescribe NSAIDs for acute gouty arthritis (E. McDonald and S. Marino; M. Harris et al.). In France, on the contrary, among 750 rheumatologists surveyed, 63% prefer colchicine, 32% prefer the combined use of this drug and NSAIDs, and only 5% prefer the isolated use of NSAIDs (S. Rozenberg et al.).
There are two alternative methods for relieving a gout attack: intravenous colchicine and the use of glucocorticosteroids.
(intra-articular, orally or parenterally) or
ACTH
.
The first report of the successful intravenous use of colchicine was published in 1954. After several years of enthusiasm for this method, it was almost abandoned due to the possibility of developing severe complications (primarily inhibition of hematopoiesis), in some cases leading to death. However, even now this method is still used, for example, in the development of severe arthritis after surgery, when other anti-inflammatory drugs are contraindicated.
It is recommended to strictly adhere to the following rules
(S. Wallace and J. Singer):
• a single dose should not exceed 2 mg, and the total dose should not exceed 4 mg (usually, 1 mg of colchicine dissolved in 20 ml of isotonic sodium chloride solution is first administered for at least 10 minutes);
• if the patient received colchicine orally the day before, this drug should not be used intravenously; after intravenous administration of a full dose, colchicine should not be used in any form for at least 7 days;
• in the presence of kidney or liver disease, the dose of colchicine should be reduced (by half if creatinine clearance is below 50 ml/min; if this figure is below 10 ml/min, colchicine is not used); in elderly patients, before intravenous use of colchicine, it is advisable to study creatinine clearance (if this is not possible, the dose is halved);
• Precautions should be taken to eliminate the risk of colchicine entering outside the vein. The onset of action of intravenously administered colchicine occurs within 6–12 hours.
It is much safer to use glucocorticosteroids. In addition to the long, although infrequently practiced, intra-articular administration of these drugs, they can be taken orally: prednisolone
in the initial daily dose of 30–50 mg. After 1–2 days, the dose is quickly reduced, and after an average of 10 days the drug is discontinued. The indication for this method of relieving a gout attack is the inability to use NSAIDs or colchicine due to intolerance to these drugs, renal failure or ulcerative lesions of the gastrointestinal tract (in the latter case, corticosteroids are administered parenterally). According to one study, oral prednisolone therapy led to improvement in all patients within 48 hours; complete disappearance of arthritis symptoms in most cases was noted on average after 3.8 days and no later than 7 days. Relapse of arthritis immediately after discontinuation of prednisolone was observed in only one case. Tolerability was good, side effects (transient hyperglycemia) were detected in only 1 of 12 patients (G. Groff et al.).
Anti-gout therapy itself
Despite many years of experience in gout therapy, two fundamental points remain not completely clear:
when to start treatment for bestophous gout, and which drug is best to choose in the absence of urate hyperexcretion.
An absolute indication for starting anti-gout therapy is the detection of tophi
(see picture).
From a practical point of view, it is advisable to classify as tophi not only subcutaneous nodules, but also destructive changes typical of gout, found on radiographs of the joints, as well as characteristic changes in the kidneys (urate nephropathy and urolithiasis). The latter is especially important, since it is kidney damage that determines the prognosis of gout
in many patients.
It is recommended to carry out appropriate examinations:
x-rays of those joints that were most often attacked, kidney studies and urine tests. It is well known that gouty nephropathy is characterized by an asymptomatic course. Therefore, it is important to pay attention to even small changes in urine tests (microproteinuria, microleukocyturia, microhematuria, and, especially, persistent sharply acidic reaction of urine - pH 4.5–5.5, with a norm of 7.4–7.5), carefully study the medical history (renal colic, pain in the kidney area, gross hematuria), do not forget to monitor blood pressure and conduct an ultrasound examination of the kidneys in search of stones.
In approximately 20% of cases, stones in patients with gout are composed of calcium oxalate and calcium phosphate. However, in most cases, a central urate “core” is detected in stones of this composition (S. Noda et al.), this explains the decrease in the incidence of calcium stones during treatment with allopurinol.
There are three different opinions regarding the time to start therapy for bestophous gout. According to the first, specific therapy should be delayed until symptomatic prophylactic treatment has been exhausted or tophi formation has been noted. This opinion is justified by the fact that tophi and chronic arthritis develop only in a minority of patients with gout.
Most experts make the prescription of anti-gout therapy dependent on the frequency of gout attacks during the year, considering the number 3-4 “critical”.
The third, less common opinion is that specific therapy should be started after the first joint attack, since even after the attack subsides, microtophus and urate crystals can be detected in the synovial membrane - a sign of chronic inflammation. However, there is no convincing evidence of the development of joint destruction in asymptomatic gout. Due to the fact that in some patients a second attack of gout may occur only many years after the first, and given the seriousness of the decision to use anti-gout therapy (lifelong nature, risk of adverse reactions), this approach to the treatment of gout is not used in practice.
Preventive anti-inflammatory therapy
Most often, it involves the daily use
of colchicine
in a small daily dose (0.5–1.5 mg). Tolerability of long-term use of colchicine in these doses is usually satisfactory; side effects (mainly diarrhea) are observed in only 4% of patients. The incidence of complications increases in case of impaired renal function. It is in these patients that depression of hematopoiesis, proximal myopathy (weakness in proximal muscle groups and increased creatine phosphokinase) and peripheral neuropathy more often develop. By 1990, 16 cases of death were known from complications of low-dose colchicine therapy. It is recommended to exercise caution in patients with impaired liver function, as well as with the simultaneous use of cimetidine, tolbutamide and erythromycin (they slow down the metabolism of colchicine).
Choosing between allopurinol and uricosuric drugs
To resolve this issue, they resort to measuring the daily excretion of uric acid.
This allows us to identify that relatively small subpopulation of gout patients in whom urate excretion is increased (more than 800 mg per day in the case of a study without dietary restrictions or 600 mg after preliminary use of a low-purine diet), which is considered a sign of overproduction of uric acid. Before this study, you should ensure normal renal function (in the case of decreased creatinine clearance, a decrease in uric acid excretion does not exclude its overproduction), and also exclude possible drug effects on the excretion of urate. It is believed that in such patients only allopurinol should be used, and uricosuric drugs are dangerous due to the increased risk of developing nephropathy and urolithiasis.
Allopurinol
.
The dose of allopurinol is selected individually and can range from 100 to 800 mg per day.
It is recommended to start therapy with a relatively small dose (100–300 mg per day), avoiding a very sharp decrease in uricemia: optimally no more than 0.6–0.8 mg% for 1 month of therapy. This helps reduce the risk of developing gout attacks after prescribing anti-gout drugs (N. Yamanaka et al.). When choosing the dose of allopurinol, you need to keep in mind that the maximum effect is achieved no later than 14 days. Side effects occur in approximately 5–20% of patients, with allopurinol discontinuation required in almost half of them. The most common are allergic skin rashes (usually maculopapular in nature), dyspepsia, diarrhea and headache. Serious complications are rare and are more common in renal failure and in patients taking thiazide diuretics. The greatest danger is represented by a symptom complex considered to reflect hypersensitivity to allopurinol: a combination of dermatitis, signs of liver damage, renal dysfunction, leukocytosis, eosinophilia or hematopoietic suppression.
Since in some patients allopurinol is the only effective drug in the treatment of gout, in the event of hypersensitivity to it, “desensitization” may be necessary, sometimes allowing therapy to be resumed. This procedure is advisable for the development of mild reactions, mainly recurrent dermatitis. Aqueous suspensions of the drug are prepared in very small concentrations (0.05 mg in 1 ml). Slowly (once every 3 days) and gradually (each time no more than 2 times) the concentrations of allopurinol are increased. The entire “oral desensitization” procedure takes about 30 days (T. Gillott et al.).
If there is no hyperuricosuria, allopurinol and uricosuric drugs are equally indicated, the choice between them being determined mainly by personal preference and the experience of the physician. Almost no objective comparisons have been made to fully weigh all the advantages and disadvantages of these two groups of funds. There is an opinion that it is preferable to prescribe uricosuric drugs to patients under the age of 60 years, with satisfactory renal function (creatinine clearance of at least 50 ml/min) and in the absence of urolithiasis.
Benzbromarone
. Benzbromarone receives the most attention for the following reasons:
• it not only enhances the excretion of urates by the kidneys (inhibits tubular reabsorption), but also inhibits the synthesis of purine bases and the absorption of uric acid from the intestine;
• its dose may not be reduced in case of moderate renal failure (unlike allopurinol);
• it is not characterized by serious adverse reactions (3-4% of patients develop diarrhea and itchy skin rashes);
• the drug is easy to use (the daily dose, usually 100–200 mg, is taken once).
The benefits of benzbromarone over allopurinol have been established in two recent studies. The first, an open-label, parallel-arm study, compared the effectiveness of benzbromarone (100 mg daily) with allopurinol (300 mg daily) in 86 men with chronic gout in the absence of uric acid hyperexcretion. With the help of benzbromarone, it was possible to achieve a more significant reduction in uric acid levels than with allopurinol treatment: uricemia decreased by 5.04 and 2.75 mg%, respectively. Improvement in renal function and the absence of new stone formation was noted only in patients receiving benzbromarone (F. Perez-Ruiz et al., 1998). It should be noted that the lack of effectiveness of allopurinol found in this study could have resulted from the use of an incomplete dose of the drug (no more than 300 mg). In terms of the degree of reduction in uricemia, benzbromarone (in a daily dose of 100–200 mg) was more effective than allopurinol (100–300 mg/day) also in patients with chronic gout in the presence of renal failure (F. Perez-Ruiz et al., 1999). Moreover, benzbromarone was effective in patients receiving diuretics (in these cases, the effect of allopurinol was clearly worse), and had a sufficient effect when allopurinol was ineffective.
Other uricosurics
Probenecid, the “oldest” uricosuric drug, is still used in the treatment of gout, with the use of which in 1949 the “era” of specific therapy for this disease began.
Probenecid
prescribed at an initial dose of 0.25 g 2 times a day. If the level of uric acid in the blood is not sufficiently reduced, the dose of the drug is increased by 0.5 g every 1–2 weeks (the maximum daily dose is 3 g). The disadvantages of probenecid are the often developing resistance, as well as the relatively frequent occurrence of adverse events (about 8% of patients have gastric dyspepsia, and 5% have allergic skin rashes). Rare serious adverse reactions include liver necrosis, nephrotic syndrome and aplastic anemia. Probenecid can prolong the effect of penicillin, cephalosporins, rifampicin and a number of other drugs, and also increases the blood concentration of naproxen and indomethacin. Acetylsalicylic acid completely blocks the uricosuric effect of probenecid.
Sulfinpyrazone
is an analogue of the metabolite of phenylbutazone, which explains the possibility of developing side effects such as inhibition of hematopoiesis and liver dysfunction, and has led to a gradual reduction in the use of this drug. The initial daily dose of sulfinpyrazone is 100 mg, divided into 2 doses throughout the day. After 3–4 days, in the absence of a sufficient decrease in the level of uric acid in the blood, the daily dose is gradually (every week) increased by 100 mg (but not more than 800 mg). The drug is able to inhibit platelet aggregation, which is valuable given the frequent presence of cardiovascular diseases in patients with gout. The most common side effect is gastric dyspepsia.
In the treatment of gout, it is possible to use a combination of allopurinol with uricosuric drugs
(usually with sulfinpyrazone or benzbromarone, but not with probenecid). This method is justified in particularly severe patients, after establishing torpidity to monotherapy. In these cases, careful selection of doses of individual drugs is required, since uricosuric drugs increase the excretion of allopurinol. A combination of individual uricosuric agents is also possible. There have been no special studies evaluating the advantages and disadvantages of such combinations of anti-gout drugs.
When prescribing both allopurinol and uricosuric drugs, two important circumstances should be remembered.
First. Due to the increased excretion of uric acid, already in the first days of using these drugs, the risk of stone formation and the development of urate nephropathy increases. In this regard, a preliminary examination of the condition of the kidneys and urinary tract is necessary (determining the level of creatinine, its clearance, ultrasound examination of the kidneys), as well as a study of urine pH. Paper analyzers, usually included with commercial citrate preparations, can be used to test urine pH. In patients with persistently low urine pH
(less than 6) before prescribing anti-gout drugs,
it is advisable to achieve its alkalization by using citrates
, sodium bicarbonate or acetozolamide (carbonic anhydrase inhibitor). These drugs are used by regularly checking the pH of the urine, the optimal level of which is 6.2–6.6. In order to prevent stone formation, it is also necessary to drink plenty of fluids (diuresis should be at least 2 liters per day). Preventive measures are taken during the entire period of selecting the optimal dose of the anti-gout drug (usually at least 1–2 months).
Second. After prescribing anti-gout medications for 6–12 months, the risk of developing gout attacks increases. Therefore, as a rule, it is recommended not to start therapy if arthritis is not yet complete.
colchicine
in small doses (0.5–1.5 mg per day) or NSAIDs for several months for prophylactic purposes The use of colchicine has been shown to prevent the occurrence of acute arthritis in approximately 85% of patients who are started on anti-gout therapy. At the same time, a number of experts express doubts about the advisability of mandatory use of preventive therapy, pointing to the relatively small risk of exacerbation of gout and the potential toxicity of colchicine.
Criteria for the effectiveness of anti-gout therapy
In the first months of therapy, the main criterion for effectiveness is the achievement of an optimal level of uric acid in the blood.
. It is no more than 6 mg% (in men), and ideally 4–5 mg%. If the concentration of uric acid does not fall below 6.8 mg%, the dissolution of urate in the extracellular fluid and tissues does not occur, and the risk of gout progression remains. After 6 months of therapy, its effectiveness is also determined by the reduction of gout attacks, the resorption of subcutaneous tophi, the preservation of renal function and the absence of progression of urolithiasis.
The list of references can be found on the website https://www.rmj.ru
References
1. Ahbern MJ, Reid C., Gordon TP Does colchicine work? Results of the first controlled study in gout. Austr. NZJ Med. 1987; 17: 301–4.
2. Gillott TJ, Whallett A., Zaphiropoulos G. Oral desensitization in patients with chronic tophaceous gout and allopurinol hypersensitivity. Rheumatology 1999; 38:85–6.
3. Groff GD, Frank WA, Raddatz DA Systemic steroid therapy for acute gout: a clinical trial and review of the literature. Seminars in Arthr. Rheum. 1990; 19: 329–36.
4. Harris MD, Siegel LB, Alloway JA Gout and hyperuricemia. Am. Fam. Physician. 1999; 15:925–34.
5. McDonald E., Marino C. Stopping progression to tophaceous gout. When and how to use urate-lowering therapy. Postgrad. Med. 1998; 104:117–27.
6. Noda S., Hayashi K., Eto K. Oxalate crystallization in the kidney in the presence of hyperuricemia. Scanning Microsc. 1989; 3:829–36.
7. Perez-Ruiz F., Alonso-Ruiz A., Calaabozo M. et al. Efficacy of allopurinol and benzbromarone for control of hyperuricemia: a pathogenic approach to the treatment of primary chronic gout. Ann. Rheum. Dis. 1998; 57:545–9.
8. Perez-Ruiz F., Calaabozo M., Fernandez-Lopez J. et al. Treatment of chronic gout in patients with renal function impairment: an open, randomized, actively controlled study. J. Clin. Rheumatol. 1999; 5:49–55.
9. Rozenberg S., Lang T., Laatar A., Koeger AT et al. Diversity of opinions on the management of gout in France: a survey of 750 rheumatologists. Rev. Rhum. 1996; 63:255–61.
10. Singer JZ, Wallace SL The allopurinol hypersensitivity syndrome. Unnecessary morbility and mortality. Arthr. Rheum. 1996; 29:82–7.
11. Talbott JH, Terplan KL The kidney in gout. Medicine 1960; 39: 405–68.
12. Wallace SL, Singer JZ Review: systemic toxicity associated with the intravenous administration of colchicine – guidelines for use. J. Rheumatol. 1988; 15: 495–9.
13. Yamanaka H., Togashi R., Hakoda M. et al. Optimal range of serum urate concentrations ti minimize risk of gouty attacks during anti-hyperuremic treatment. Adv. Exp. Med. Biol. 1998; 431:13–8.
14. Yu: TF., Gutman AB Uric acid nephrolitiasis in gout: pridisposing factors. Ann. Intern. Med. 1967; 67:1133–48.
15. Yu: TF. Urolitiasis in hyperuricemia and gout. J. Urol. 1981; 126:424–30.
| Applications to the article |
| Rome criteria for the diagnosis of gout: 1. Hyperuricemia (uric acid in the blood more than 7 mg% in men and more than 6 mg% in women) 2. Presence of gouty nodules (tophi) 3. Detection of urate crystals in synovial fluid or tissues 4. A history of acute arthritis, accompanied by severe pain, which began suddenly and subsided within 1-2 days The diagnosis of gout is considered reliable if at least two signs are detected. |
| Tophus on the ear |
Metabolic effects of antihypertensive drugs
Transcript of the author's broadcast by Professor Sergei Rudzherovich Gilyarevsky, held on February 29, 2012.
Drapkina O.M.: - Hello, dear colleagues! The program “Evidence-Based Cardiology” is on air, and its author, and leading professor Gilyarevsky, well, and such co-leading professor Oksana Mikhailovna Drapkina.
Gilyarevsky S.R.: - Good evening, dear colleagues.
Drapkina O.M.: - Hello! It turns out that we have been going on air at this time for many years. The gear ratio has been slightly changed. Well, today, Sergei Rudzherovich, we will probably talk about metabolism, metabolism in general.
Gilyarevsky S.R.: — Even, maybe not in general, but we will touch upon such a seemingly very banal topic as the metabolic effects of antihypertensive drugs. This topic, it seemed, is discussed at every conference, and everyone who represents a certain class of antihypertensive drugs always says that this class is the most metabolically neutral, and in general, when they discuss the problem, they usually still evaluate or discuss the effect antihypertensive drugs on blood glucose levels, on the risk of developing diabetes mellitus and on blood potassium levels, on electrolyte disturbances, especially diuretics.
Drapkina O.M.: — Can this be called pleiotropic effects?
Gilyarevsky S.R.: - Well, it’s hard to say...
Drapkina O.M.: - Well, multiple...
Gilyarevsky S.R.: - Well, these are more likely side effects, not pleiotropic ones.
Drapkina O.M.: - But if they improve insulin sensitivity.
Gilyarevsky S.R.: — And, if they improve insulin sensitivity, probably yes. It seems to me that when we talk about antihypertensive drugs, it is still very important to talk first of all about their safety. Indeed, it is difficult not to agree that there are some pleiotropic effects in some drugs, which still sometimes make one wonder and discuss what we have done.
I would like to turn to the results of a meta-analysis, because when side effects are discussed, they very often resort to meta-analysis, because if clinical effects are quite well identified during large randomized trials, then the statistical power of even large studies is not always sufficient in order to detect certain side effects. And therefore, in this situation, they often turn to meta-analyses or some large observational, population studies.
A well-known meta-analysis that focused on the effects of antihypertensive drugs and their impact on the risk of developing diabetes mellitus. And compared with diuretics, which are known to most increase the risk of developing diabetes, other drugs had a lesser effect on the risk of developing diabetes, including placebo, and, of course, the most positive effect, the greatest, was observed in receptor blockers, sartans and ACE inhibitors. Moreover, you can even remember that one of the sartans, valsartan, in the NAVIGATOR study, it was even proven that its use in people with prediabetes leads to a reduction in the risk of developing diabetes by 14%.
Therefore, questions of the effect of antihypertensive drugs on metabolic parameters concern, in general, everyone. And in this regard, the informational reason for our today's program is the publication of a very large case-control study, which was based on the analysis of a real database, a population database of the United Kingdom, in which data on the incidence of gout and in general were recorded. different diagnoses, including in patients with arterial hypertension who received different antihypertensive drugs.
And in the course of this study, they tried to answer the question of how different drugs affect the risk of developing gout. That is, this was the main goal of this analysis. Here, perhaps, we need to remember what role uric acid plays in general as a risk factor for the development of cardiovascular diseases and, it would seem, from our institute years we know well that gout is hyperuricemia, it is a risk factor for cardiovascular diseases. However, in 1999, the results of the Framingham study were published, which questioned the independence of gout and hyperuricemia as a risk factor for the development of cardiovascular diseases. Perhaps this was due to the fact that this study included individuals who had a fairly high risk of developing complications of cardiovascular diseases, and therefore hyperuricemia and gout no longer added something as a risk factor, but, nevertheless, later they were Several studies have been published, most of which provided evidence that hyperuricemia is an independent risk factor for the development of cardiovascular diseases, but there is still no exact answer.
But, be that as it may, if hyperuricemia is not even an independent risk factor for the development of cardiovascular diseases, then at least the development of gout is certainly a very unpleasant disease that worsens the patient’s quality of life and requires the use of additional medications , well, it is often accompanied by pain, hyperuricemia worsens kidney function. So here, really, if it is possible to prevent the development of gout, then regardless of whether it is an independent risk factor or not, this is very important.
Drapkina O.M.: - You just said, Sergei Rudzherovich, I remembered 1999 there, this is understandable, our student life, even earlier. But this year we celebrate the 180th anniversary of the birth of Sergei Petrovich Botkin, and in his clinical lectures, this was 1867, he said that hypertension is associated with an image with a lot of uric acid. Apparently this was already determined then. I remember now, could this be defined then in lectures?
Gilyarevsky S.R.: - But at least they could have a crystal.
Drapkina O.M.: - Moreover, with his such excellent propaedeutic qualities, he watched urates and tophi. Georgy Fedorovich Lang said the same thing, and Myasnikov, that is, about gout in general...
Gilyarevsky S.R.: - So in our real practice, in patients with gout, they very often had coronary heart disease, but the question arises: in whom an increase in the level of uric acid is accompanied by a greater influence on the risk of developing complications of cardiovascular diseases - in men or women.
Drapkina O.M.: — It seems to me that it’s in men.
Gilyarevsky S.R.: - But as often happens, it seems one at a time, but in fact, it turns out that it’s in women. This is probably due to the fact that in men the risk of complications of cardiovascular diseases is higher, so, probably, hyperuricemia is already dissolved in this. And by the way, in the LIFE study, where we recall that losartan is compared with atenolol, it was in women that the influence of uric acid levels was greater as a risk factor for the development of complications of cardiovascular diseases. So, in any case, such a problem exists, and it was partly solved during the implementation of this study. Very large studies: data were included on almost 25,000 newly diagnosed gout patients and 50,000 demographically matched controls who...these data were obtained from a UK online health database. And, moreover, during this analysis, even in order to clarify whether there were any errors, because doctors can make a diagnosis of gout, well, just like that. Some people see something wrong with the finger, arthrosis, Heberden’s nodes, but it seems to them that that’s all...
Well, of course, I’m exaggerating everything, but mistakes can happen if they are not based on a thorough analysis, on the determination of uric acid there. Therefore, here, for clarification, an additional analysis was performed, which included data only from patients who received treatment for gout. And yet, the results turned out to be about the same; in fact, the results were somewhat sensational. It turned out that calcium antagonists reduce the risk of developing gout by 13%. Well, the leader here turned out to be losartan, an angiotensin II receptor blocker, diuretics, these are natural results, diuretics more than doubled the risk of developing gout, beta blockers by 150%, that is, one and a half times, ACE inhibitors by 1.2% and angiotensin II receptor blockers – increased, yes.
Drapkina O.M.: - So this is a directly unique effect of losartan?
Gilyarevsky S.R.: - Yes, a unique effect, and indeed there is evidence that the severity of the uricosuric effect of losartan may be similar to that of the classic uricosuric drug probenecid, which is well known, that is, this effect exists. And they even tried to establish the reasons for this, the reasons for this effect, and it became obvious that this effect is not associated with angiotensin II receptor blockade, because, as we saw in the course of performing this analysis, other angiotensin II receptor blockers did not lead to a decrease, even by In their background, there was an increase in the risk of developing gout, including such well-known drugs as valsartan, which reduced the risk of developing diabetes, telmisartan, and candesartan. Only losartan.
Similarly, taking ACE inhibitors was also not accompanied by a decrease in uric acid levels. So, in the course of several experimental studies, it was shown that the use of losartan leads to blockade of the uric acid transporter in the kidneys - URAT-1, and thus the action of this transporter in the apical part of the tubular cells is suppressed due to direct inhibition, which is also typical for other uricosuric drugs agents such as probenecid and benzbromarone.
Drapkina O.M.: — There was, of course, a clawed frog.
Gilyarevsky S.R.: - Well, I looked on the Internet when I saw this, there really is such a frog, about its biological characteristics. For some reason she is a convenient model for studying URAT-1. So this is a unique action. Here, of course, the question arises, if... by the way, you didn’t specify, what is meant by hyperuricemia? What is the concentration of uric acid?
Drapkina O.M.: - It depends on what units it is measured in.
Gilyarevsky S.R.: — It is believed that approximately 600, about 647 mg per deciliter, this is the concentration of uric acid that precipitates at a temperature of 37 degrees, i.e. Crystal formation occurs precisely at this level of uric acid. I think it's 6.7 mg per deciliter. This is taken as the starting point.
By the way, one study showed that an increase in uric acid concentration above this level compared with a level less than 4.3 is accompanied by a statistically significant increase in the risk of heart failure by approximately 50% and the risk of stroke, but not coronary heart disease. That. So this is what you need to pay attention to.
The question arises: okay, why is it necessary to give a hypertensive drug, which in addition exhibits its pleiotropic effect, reduces the level of uric acid. Isn’t it better to give the patient a normal drug for the treatment of gout: allopurinol, which we talked about, or probenecid and reduce the level of uric acid. But still, it is believed that today such a concentration - asymptomatic hyperuricemia, even at a concentration of 6.7 - is not an indication for the prescription of allopurinol, that is, in my opinion, it should be a level of about 900 mg per deciliter or should be clinically overt gout.
So, let’s say, for primary prevention in the development of gout, these drugs are not very recommended for moderately elevated uric acid levels. In general, as a rule, in real practice you see, well, 500, well, 400-something. Therefore, of course, the role of losartan here is very high. Of course, when we talk about the use of losartan, we probably need to remember a number of studies and, in general, the evidence-based history of this drug. It started very interestingly with the ELITE study, and then in this study - it was not very large - there were, in my opinion, less than 1 thousand patients, and they wanted to see how much better losartan would be tolerated than captopril.
It was better tolerated in patients with heart failure, but unexpectedly it was found that it statistically significantly reduced the risk of deaths, including sudden death, compared to captopril, and then it seemed that the end of the era of ACE inhibitors had come, sartans would solve all problems, associated with the effect on the renin-angiotensin system.
But then there was the ELITE-2 study, a larger study, the study had statistical power to detect the risk of adverse outcomes, including mortality, and it failed, and captopril even had some advantages over losartan. Further history, also at least in heart failure, the HEAAL study was performed, which compared a dose of 150 mg and 50 mg, that is, the dose that was no better than captopril - and they proved that when using such a high dose of losartan there are statistically significant improvements in forecasts. Therefore, probably, in the treatment of arterial hypertension, a dose of 100 mg should still be used more often, and in case of heart failure, even formally, higher doses should still be used, naturally taking into account the level of potassium in the blood. So for the treatment of arterial hypertension this, of course, is of great importance. So the drug is not always the first of a certain class, it loses its relevance, this is interesting, because this is also the development of evidence-based medicine.
Drapkina O.M.: - Sergey Rudzherovich, does this mean, according to the data that you also show, the level of uric acid, well, let’s assume, does not exceed eight, so be it - it will be more than seven, but nine. Hypertensive, obese, say, high-risk hypertension, taking into account the effect on uric acid levels, a combination of losartan with a calcium channel blocker would probably be preferable for such a patient.
Gilyarevsky S.R.: - Yes, of course, we haven’t really discussed the effect of calcium antagonists, but they also have this possibility, so this idea of prescribing amlodipine with an ACE inhibitor or sartan is good. And from this point of view, the combination of amlodipine and losartan is probably additive in individuals with a tendency to hyperuricemia. This study did not evaluate the effects of combined use of calcium antagonists and losartan, but...
Drapkina O.M.: - But we don’t have such a fixed combination.
Gilyarevsky S.R.: — For losartan, as far as I know, no. So this is also a reason to think.
Drapkina O.M.: — We should probably contact you, dear colleagues, if you have questions, write to us in the chat, we will answer. Take advantage of the moment, and if there are no questions or they come later, we will also answer in the next programs, yes, Sergei Rudzherovich?
Gilyarevsky S.R.: - Yes.
Drapkina O.M.: - Well, maybe we should announce what will await our viewers, our colleagues in the next program?
Gilyarevsky S.R.: - Yes, we can announce it. We wanted to discuss a problem that also seemed to have already been exhausted, the role of coronary artery stenting in the treatment of coronary heart disease, but there are also some informational reasons to return to the discussion of this problem.
Drapkina O.M.: - We are waiting for you on Wednesday. See you.
Gilyarevsky S.R.: - See you later. Goodbye.