It is believed that primary disturbances in the structure of the intervertebral disc occur in the nucleus pulposus, which normally has high turgor due to the large amount of water present in bound form and included in the structure of proteoglycan molecules [1, 2]. Such high hydrophilicity of the nucleus pulposus determines the necessary shock-absorbing properties of the disc and ensures its high resistance to vertical loads. In this case, the maximum biomechanical load is first perceived by the nucleus pulposus, as a highly specialized structure, and then the load is distributed to the plates of the annulus fibrosus, starting from its inner layers [3]. The gradual damping of the biomechanical load is due to the sequential stretching of the collagen fiber bundles of the fibrous ring.
However, according to many authors, a key role in triggering the mechanisms of disc degeneration is played by structural and metabolic disorders of the cartilaginous endplate (CLP) [4–8].
The purpose of this work is to conduct an analytical review of literature data on the role of damage to the cartilaginous endplate in the pathogenesis of degenerative disorders of the intervertebral disc.
Structure of the cartilaginous endplate
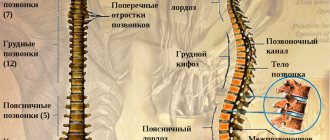
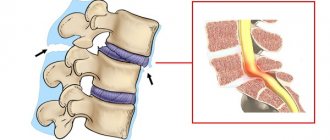
The functional unit of the spine is the vertebral motion segment (VMS), consisting of 2 adjacent vertebral bodies and the intervertebral disc located between them. The MDS is considered as a modified joint in which the role of articulating bones is played by the bodies of adjacent vertebrae, the cartilaginous endplates are a kind of analogue of the articular surfaces, the nucleus pulposus plays the role of synovial fluid in the joint cavity, and the fibrous ring plays the role of the joint capsule [9–12].
Since the joint cannot function without articular surfaces, then, by analogy, the vertebral motor segment cannot function without cartilaginous endplates, which delimit the bodies of adjacent vertebrae from the highly hydrated nucleus pulposus and prevent its protrusion into adjacent vertebral bodies. When the cartilaginous endplate is damaged or degenerated, the “tightness” of the nucleus pulposus is broken and it comes into contact with bone tissue, and pro-inflammatory cytokines are released - tumor necrosis factor a (TNFa), interleukins, which induce bone destruction.
Most authors consider the cartilaginous endplate as a component of the intervertebral disc [13–18], along with the nucleus pulposus and the annulus fibrosus. Other researchers consider it to be part of the vertebral body [19, 20], some authors consider it as an independent anatomical formation—a transitional structure [21, 22] that separates the tissue of the intervertebral disc from the vertebral bodies.
Although the cartilaginous endplate is an integral component of the spine, its structure differs significantly between humans and animals. In animals of various species, CKD has specific structural features. Apparently, this is due primarily to the specifics of the biomechanical load placed on the spine, especially with the presence or absence of vertical load, which is most pronounced during bipedalism.
Thus, in humans, the cartilaginous endplate is represented by hyaline cartilaginous tissue of a typical structural organization [15]. It consists of a large number of layers of chondrocytes and a matrix that contains predominantly type II collagen and proteoglycans. The collagen fibers of CKD are arranged horizontally. The fibrils of the inner plates of the annulus fibrosus are woven into the hyaline cartilage matrix of the endplate, separating the intervertebral disc tissue from the spongy bone of the vertebral bodies [14]. In this way, a “closed package” is formed, which closes the nucleus pulposus into a continuous fibrous frame, represented along the periphery by a fibrous ring, and above and below by hyaline plates, forming a single system of fibers.
To date, there is no definitive opinion regarding the blood supply to the cartilaginous endplate. The prevailing view is that CKD in healthy adults, like any hyaline cartilage, does not have vessels and nerves [14, 17, 18, 23, 24]. However, a number of researchers indicate the presence of vessels in the cartilaginous plate [25–28]. These contradictions appear to be related to studies of the endplate at different ages.
As is known, trophism of the disc is carried out due to the actively occurring processes of diffusion of molecules and ions into the disc tissue from the cartilaginous endplates. In this way, anabolic processes are carried out and, accordingly, a reverse outflow of catabolic products occurs. However, as some data have shown [25, 26, 28], the cartilaginous endplate (especially in its central section) contains a network of tiny capillaries, which were identified as a result of injection of a contrast agent into the intraosseous vessels. It is these capillaries that play a key role in the supply of nutrients to the disc [29, 30]. With age or during the process of degeneration of the hyaline endplate, accompanied by its petrification and ossification, these capillaries become calcified.
In children, the cartilaginous endplates are much thicker than in adults. According to PP Raj (2008) [24], they contain many vascular channels passing through the annulus fibrosus and nucleus pulposus. In adults, the cartilaginous endplates are thin, with single vascular channels passing through them, or they are completely absent. Adult CKDs typically have a thickness of less than 1 mm, which varies within each individual disc and tends to be thinnest in the central region adjacent to the nucleus pulposus [31, 32].
In humans, the anteroposterior diameter of the endplate increases sequentially from the cervical to the lumbar region, while in tetrapods such as the calf, sheep and pig, its diameter remains the same throughout the entire spine [33]. In this case, the endplate has a slightly larger diameter in the cervical spine and a smaller diameter in the lumbar spine, with the exception of that in the calf, in which the diameter of the lumbar endplate is similar to that in humans. In the lumbar region of primates (orangutan, chimpanzee) and kangaroo, the diameter of the endplate is smaller than in humans [34].
The endplate in a rabbit consists of an outer layer of hyaline cartilage and an inner layer of bone tissue, which most often directly borders the outer plates of the fibrous ring, less often separated from them by a thin layer (2-3 rows of cells) of hyaline cartilage. This layer is not found in all disks and only in its middle part. This layered structure of the endplate, characteristic of rabbits, distinguishes it from that of humans, which consists only of hyaline cartilage [16].
The cartilaginous layer of the rabbit endplate is formed by chondrocytes typical of hyaline cartilage, surrounded by a wide capsule that form columns. The intercellular matrix is homogeneous, basophilic when stained with hematoxylin and eosin. When stained with toluidine blue, the cartilage matrix has metachromasia, which indicates a high content of proteoglycans in it. The bone layer of CKD has a structure identical to the bone tissue of the vertebrae: spongy bone with wide trabeculae forming cavities filled with bone marrow [16].
According to DM Elliott [35], the chemical composition [36], structure, biomechanical properties [37, 38] and the nature of death of notochordal cells [39] of the rat intervertebral disc are similar to those in humans (taking into account the differences in their geometric parameters: height and disk area). This indicates that the characteristic organization and properties of disc tissue are preserved in representatives of different animal species and humans.
According to some authors, the rat does not have a cartilaginous endplate. Other authors point to its presence [40, 41], but point out the distinctive features of its organization. AJ Hayes et al. (2011) [41] studied the embryogenesis of intervertebral disc components, including the cartilaginous endplate, and analyzed its structure in adult Wistar rats. The authors showed that collagen fiber bundles from CKD rats are embedded in the inner layer of the annulus fibrosus, forming a collagen scaffold around the nucleus pulposus. The cartilaginous endplate in rats is thin, represented by several layers of collagen fibers and chondrocytes located between them. Moreover, the structure of CKD itself resembles fibrous cartilage, and is not hyaline cartilage, as in humans.
Age-related changes in the cartilaginous endplate and its changes during degeneration
Degeneration of the cartilaginous endplate begins in the area of its contact with the nucleus pulposus, which leads to disruption of the balanced process of substances entering the disc [3] and the reverse outflow of unutilized metabolic products. This leads, on the one hand, to degeneration of the cells of the nucleus pulposus, and on the other, to the accumulation of catabolic products in it and subsequent destruction.
In children aged 3 to 10 years, a decrease in vascular density in the cartilaginous endplate was noted. On the other hand, the number of obliterated vessels increases with age. The first cracks appear in the endplate. These changes are most clearly visible in its center [18]. In the endplate of adolescents (11–16 years old), a decrease in the number of blood vessels is observed against the background of progressive obliteration of existing ones, which has an adverse effect on maintaining the structural integrity of the disc matrix. A decrease in the lumen and number of blood vessels correlates with a significant increase in the extent of areas of cartilage disorganization, a decrease in cellular density and the appearance of cracks in it.
Endplate injuries at the age of 17–20 years are similar to those occurring in the previous group. N. Boos. et al. (2002) [18] noted the presence of vascular canals in the human cartilaginous endplate until the third decade of life. Vascular reduction was accompanied by progressive thinning of the endplate and microscopic protrusions into the adjacent subchondral bone. From 20 to 30 years of age, endplate disorders are similar to those noted in younger groups, but they are more numerous.
Ages 31–50 years are characterized by a significant increase in the frequency and extent of endplate disorders. In the fourth decade, its disorganization leads to the formation of numerous cracks and fissures, as well as mucoid and granular changes in the matrix. At the age of 51–70 years, endplate tissue disorders are most pronounced. During the sixth and seventh decades, the endplate is replaced by fibrocartilage with areas of focal necrosis. In old age, CKD degeneration may be associated with vascular invasion and replacement of calcified cartilage with bone tissue, which leads to a reduction in the supply of substances and degeneration of the nucleus pulposus [15]. Microfractures and bone sclerosis were noted in the ossified endplate. Some researchers have noted a correlation between cartilaginous endplate sclerosis and disc degeneration [32, 42, 43]. In elderly people over 70 years of age, disorganization of cartilage and bone tissue in the endplate remains at the level of disorders noted in the previous group [18].
It should be noted that age-related degeneration of the intervertebral disc occurs in humans and animals that maintain an upright body position for a long time. In rats, intervertebral disc degeneration is a rare phenomenon. However, in sand rats that spend significant time standing on their hind limbs, radicular syndrome occurs in adulthood (18 months). More than 50% of animals show morphological signs of intervertebral disc degeneration in the lumbar spine [44, 45], which indicates a relationship between the process of disc degeneration and bipedalism. In this regard, sand rats are a successful natural model for studying degenerative diseases of the spine.
When studying (for 40 weeks) a puncture model of osteochondrosis in rabbits, obtained by partial-thickness puncture of the fibrous ring [16], pronounced changes were found in the hyaline layer of the endplates of intact intervertebral discs (adjacent to damaged ones), which began from the 6th week of the experiment and then progressed. There was necrosis, destruction, calcification, ossification, thinning and fragmentation of hyaline cartilage, accompanied by a reparative reaction. By the 40th week, some of the discs contained only isolated fragments of cartilage surrounded by bone tissue. Similar changes, especially ossification of CKD, have been noted with aging and intervertebral disc degeneration in humans [46].
Causes of subchondral osteosclerosis of the spine
Subchondral osteosclerosis of the spine develops for various reasons. These are mainly age-related degenerative processes that develop against the background of impaired blood supply to certain parts of the body. Gerontological changes are more typical for older people over 70 years of age.
In young patients, there are other causes of osteosclerosis of the endplates of the vertebral bodies. They include the following negative influence factors:
- deformation of the vertebral bodies as a result of salt deposition and the formation of osteophytes;
- curvature of the spinal column in the form of hyperkyphosis, hyperlordosis or scoliosis;
- dystrophic changes in the muscle tissue surrounding the spinal column;
- disruption of the process of blood microcirculation and diffuse exchange with the tissues of the spinal column;
- consequences of traumatic effects (impacts, fractures, subluxations, sprains of the ligamentous apparatus, muscle fiber ruptures, etc.);
- injury to the spine during the passage of the fetus through the birth canal;
- genetic predisposition factors (if there are established cases of osteosclerosis in the family, then you should be careful about the timely diagnosis of the pathology);
- physical inactivity or leading a lifestyle with predominantly sedentary work and lack of regular sufficient physical activity on the muscular frame of the back;
- heavy physical labor, accompanied by constant lifting of weights exceeding the actual capabilities of a person;
- tumor processes in the spinal column;
- various infectious and inflammatory processes.
Until recently, it was believed that osteochondrosis was the direct cause of osteosclerosis of the vertebral bodies. But thanks to numerous scientific studies, the opposite relationship between these two pathologies has been identified. In fact, osteosclerosis of the endplates of the vertebral bodies is a direct risk factor for the development of osteochondrosis at a young age.
The role of apoptosis in the pathogenesis of cartilage endplate degeneration
Currently, more and more data are emerging on the connection between disc degeneration and chondrocyte apoptosis [47–53]. In this case, chondrocyte apoptosis is primarily activated in the cartilaginous endplate [54, 55]. It is believed that it is preceded by a violation of the structure of the cartilaginous matrix, which results in the separation of chondrocytes and disruption of their trophism. It is known [1] that not only chondrocytes influence the structure of the cartilage matrix, but there is also feedback when the regulatory function of the matrix on the structure of the cell takes place. Destruction of the matrix leads to disruption of interaction at the “matrix-cell-matrix” level, while disruption of the structure of the cartilage matrix causes structural and then functional changes in chondrocytes, which, in turn, leads to disruption of the biosynthesis of matrix components by such destructively altered cells.
Disruption of the matrix-cell interaction induces pathological apoptosis of chondrocytes, when cell death occurs in a shorter period than it is physiologically programmed. The process of pathological apoptosis of chondrocytes of the cartilage endplate involves signaling molecules - members of the tumor necrosis factor superfamily - FasL (Fas ligand) and TRAIL (TRAI ligand) and their pairs (Fas ligand system with Fas receptor, TRAIL/DR4/DR5 system) [50, 52, 56] . This ensures the connection of the apoptosis ligand and its receptor on the chondrocyte membrane [57], which leads to the induction of apoptosis.
Activation of the apoptosis process takes place both in the cartilaginous endplate and in the tissues of the nucleus pulposus and annulus fibrosus. However, the earliest indicator of disc degeneration is the activation of apoptosis specifically in CKD [8]. As a result of disruption of the endplate structure and blocking the diffusion of nutrients into the disc, apoptosis progresses in the chondrocytes of the nucleus pulposus and then the annulus fibrosus. It is in discs with a high degree of degeneration that the highest frequency of chondrocyte apoptosis was recorded, which was revealed by assessing the expression of cell death receptors DR4 and DR5 (death receptor4 and DR5) and their ligand TRAIL (TNF-related apoptosisinducing ligand - tumor necrosis factor-related apoptosis-inducing ligand) , which is a trigger for apoptosis. The expression of these receptors correlated with the progression of degenerative changes in the intervertebral discs [49]. According to some data [48], with degeneration of the intervertebral disc, there is not an increase, but a decrease in the expression of Fas ligand in the cells of the nucleus pulposus.
In this regard, it should be noted that there has been significant progress in the field of genetic engineering, which makes it possible to regulate the expression of apoptosis genes in nucleus pulposus cells at the level of blocking Fas ligand expression using the RNA interference method [50]. The use of short double-stranded interference RNA (short interferense RNA - siRNA) leads to disruption of RNA transcription (inactivation of Fasligand mRNA) or subsequent translation, which ultimately leads to inhibition of apoptosis of nucleus pulposus cells.
Wu Jingping et al. (2010) [40] examined the effects of age and weight bearing on cartilaginous endplate morphology and chondrocyte apoptosis in rats. To do this, they created a model of bipedal rats by amputating the forelimbs (45 animals) and special conditions for keeping the animals. Intact rats (n = 40) of the same age served as controls. When the animals reached 3, 6, 9 and 12 months, 8 rats from each group were killed. Paraffin sections (stained with hematoxylineosin and TUNEL) prepared in the sagittal plane at the L4–L5 level of the spine were analyzed. The number of apoptotic and viable cells in cartilaginous endplates and their thickness were counted, and the degree of CKD degeneration was assessed. Apoptosis was initially detected in the cartilaginous endplate. It increased with age and led to a marked decrease in cell density. The rate of chondrocyte apoptosis in CKD 6-month-old bipedal rats was significantly higher than in control group animals. When comparing the performance of bipedal rats 6 and 9 months of age, a statistically significant increase in the rate of apoptosis was noted in animals of the older age group (P < 0.05). A negative correlation was found between the number of viable cells of the cartilaginous endplate and the degree of its degeneration (r = –0.97, P < 0.05). At the same time, the group of bipedal rats had a more severe degree of CKD degeneration, a more pronounced thickening of its calcified layer and more structural defects of the cartilage. Based on these data, the authors [40] conclude that vertical weight bearing is a key factor in increasing chondrocyte apoptosis and cartilage endplate degeneration.
Experimental injuries to the cartilaginous endplate
Experimental models that reproduce damage to the cartilaginous endplate make it possible to trace the degenerative changes occurring in the intervertebral disc. Many experimental models have demonstrated the role of traumatic injury to the cartilaginous endplate in the progression of disc degeneration [4–8].
W. C. Hutton et al. [58] modeled disc trophism disruption in dogs by injecting bone cement into the area of the vertebral body adjacent to one or two cartilaginous endplates. After 70 weeks, they found degenerative changes in the experimental discs compared to control discs of the same animals. In an experiment on sheep, the blood supply was disrupted in the endplates of the intervertebral discs of the lumbar spine [59], then the restoration of vascularization was prevented using titanium foil. It was shown that this method of intervention led to a significant reduction in the number of vascular buds approaching the nucleus pulposus of the disc [60].
In an experiment on 6 domestic pigs, S. Holm et al. [5] developed a model of disc degeneration that mimics that in humans. For this purpose, animals underwent perforation of the cranial cartilaginous endplate with penetration of the hole into the nucleus pulposus. 3 months after surgery, compression properties were studied at the level of the L2–L4 motor segments, and intradiscal pressure in the damaged discs was also measured. A significant decrease in water content was observed in the outer portion of the annulus fibrosus on the ventral side of the spine. Intradiscal pressure of the nucleus pulposus was significantly reduced.
Histological and histochemical studies have shown a marked decrease in proteoglycan content in the nucleus pulposus, as well as a decrease in cell density in this area. The nucleus pulposus lost its gel-like structure and did not have a characteristic color, and lamination of the plates was observed in the fibrous ring.
S. Sobajima and DG Anderson [4, 6], using a model of wound damage to the annulus fibrosus in rabbits, showed that the onset of disc degeneration is associated with pathological gene expression. It was found that after disc transection, the gene expression of type II collagen and aggrecan, as well as the tissue inhibitor of metalloproteinase TIMP1, was decreased [6], while the expression of type I collagen was increased. This is confirmed by data [4] on increased gene expression of collagenases MMP1 and MMP13.
The works of many authors emphasize that static mechanical load is one of the important factors inducing apoptosis of chondrocytes [8, 53, 61]. A 24-hour organ culture of intervertebral discs and cartilaginous endplates of the coccygeal spine of mice showed that with increasing load (0; 0.2; 0.4; 0.8; 1.0 μPa), the number of apoptotic cells in the disc increased, especially in areas of CKD [53].
The TUNEL method revealed an increase in the frequency of cell apoptosis in the intervertebral discs of the caudal spine of mice, which was observed during static compression of the discs (above 1 μPa) using special devices [61]. The incidence of chondrocyte apoptosis correlated with the duration and intensity of compression applied.
In an experiment on 40 mature New Zealand rabbits [8], the animals were exposed to the upper and lower edges of the C4 and C5 vertebral bodies and the upper edge of the C6 vertebra. Bone cement was injected from the dorsal side along the exposed surface of the vertebral body in order to disrupt the nutritional pathways of the cartilaginous endplate, and under these conditions its structure was assessed. After 4 and 8 weeks, chondrocyte apoptosis was identified using the TUNEL method in cartilage endplate samples, and type II collagen expression was determined in intervertebral discs using immunohistochemistry. A significant increase in the number of apoptotic cells in the cartilaginous endplate was shown at both observation periods. A strong negative correlation was found between the frequency of endplate chondrocyte apoptosis and type II collagen expression. Apoptosis of cartilage endplate chondrocytes leads to decreased expression of type II collagen in the intervertebral disc and causes its degeneration. A similar dependence was confirmed by F. Wang [62] on a primary culture of cartilaginous endplate chondrocytes, who showed a decrease in cell viability with an increase in the frequency of apoptosis.
D. Haschtmann et al. [7] were the first to develop an experimental model of traumatic injury to the intervertebral disc in vitro. To do this, in an organ culture of samples including a cartilaginous endplate and an intervertebral disc of 4 mature Burgundy rabbits, a special device was used to rupture the endplate with an applied rupture energy of 1.2 J. This in all cases led to the formation of a herniation of the nucleus pulposus through the damaged endplate . After 9 days of culture, the disc was analyzed for the presence of necrotic or apoptotic cells and determined the expression of proapoptotic genes, as well as other genes involved in the degeneration process, such as matrix metalloproteinase genes. Cell damage was assessed by measuring lactate dehydrogenase (LDH) activity. A significant increase in LDH activity was found in the test samples compared to the control. In addition, there was a consistent increase in caspase3 gene expression throughout the disc. The expression of proapoptotic proteins such as FasL and TNFa was increased in the nucleus pulposus, while transcripts of metalloproteinases MMP1 and MMP13 (collagenases) were increased in all structural regions of the disc.
These studies showed that cartilaginous endplate tears lead to both necrotic and apoptotic cell death of the nucleus pulposus and annulus fibrosus. Moreover, expression of the proapoptotic proteins FasL and TNFa by nucleus pulposus cells can lead to the progression of apoptosis induced by cellular Fas and TNFa receptors. Overexpression of TNFa and MMPases may damage disc metabolism. The rapid biological response of the disc to endplate injury has revealed how intervertebral disc degeneration can be initiated.
Fragments of the cartilaginous endplate in postoperative material from patients with disc herniation
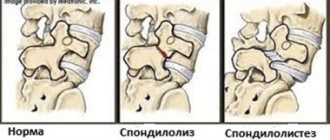
It is traditionally believed that when the cartilaginous endplate is damaged or degenerated, Schmorl’s hernias form [9]. In this case, through the formed defects (ruptures) of the hyaline endplate, the nucleus pulposus penetrates into the body of the vertebra adjacent to it. Schmorl's hernias are asymptomatic in most cases. In a study of autopsy specimens, RC Hilton et al. [63] found that Schmorl's hernias were observed in more than 70% of cases, with equal frequency in people over and under 50 years of age, indicating that they occur relatively early in life.
However, the observations of many researchers [64–68] indicate that ruptures of the cartilaginous endplate, against the background of its severe degeneration, can often lead not only to Schmorl’s hernias, but also to the formation of so-called rupture-type hernias, in which extrusion epidural and subdural [68] or sequestered hernias [65, 67] contain fragments of cartilaginous tissue. One of the important conditions for the occurrence of hernias of this type is the presence in the patient of a dorsal angular defect of the vertebral body [68]. Against this background, ruptures of the cartilaginous endplate in an area equal to 1/3 of its length on the dorsal side of the spine lead to protrusion of the hernial material in the direction of the spinal cord canal. When comparing MR images of two groups of patients with an angular defect of the dorsal region of the vertebral bodies, it was found that in the group of patients with an unpronounced angular defect, cartilaginous material was present in 40% of cases, while in patients with a pronounced defect - in 82% (P = 0.019 according to Fisher) .
This type of hernia most often occurs in middle-aged people (31–45 years old). In young individuals, its frequency of occurrence increases with age, and in elderly and senile individuals it decreases. There is also a direct relationship between the weight of postoperative hernial masses (in grams) and the presence of cartilaginous endplate fragments in them [68].
According to some authors [66], in herniated samples of the cervical spine of mature and elderly people, fragments of hyaline and fibrous cartilage were present, and herniation of the cartilaginous endplate was the predominant type of herniation in these areas. Similar results were obtained in the lumbar spine [64] in a detailed study of 100 patients with disc herniations. It was found that in 44% of cases, the free hernia fragments contained mainly endplate material, and in 54% of patients, nucleus pulposus.
G. Schmid et al. [68] showed that approximately 50% of patients had a rupture type of hernia formation in the lumbar spine at the level of L4–L5 and L5–S1. In this case, the extruded hernia material contained hyaline cartilage of the endplate, the amount of which was 5–50% of the total volume of the hernial masses. Using MRI studies, a correlation was established between the intensity of signal changes in the middle part of the endplate and the presence of cartilage material in the herniated disc of patients. The relationship between the amount of cartilaginous material in a disc herniation and the degree of hyaline endplate degeneration supports the hypothesis that hyaline plate tears are the source of rupture-type disc herniation.
These data are in good agreement with the data of M. Tanaka et al. [65] that most disc ruptures occur in its internal or intermediate sections at the interface between the annulus fibrosus and the hyaline endplate. The rupture type of herniation is also associated with the formation of concentric and radial cracks of the fibrous ring and predominates in elderly people. However, this type of herniation can also occur in a significant percentage of younger patients, especially if they have a large disc herniation.
Clinical observations have shown that in autopsy discs with severe degeneration and ruptures of the annulus fibrosus with prolapse of the nucleus pulposus, ruptures of the hyaline endplate were found [65]. The authors found that there is a weak connection between the cartilaginous endplate and the subchondral bone, while the connection between the cartilaginous endplate and the internal fibers of the annulus fibrosus is strong. After autopsy studies, it was found that fragments of the hyaline endplate with fibers of the annulus fibrosus attached to it can separate from the vertebral body and form a sequestered hernia [65, 67]. This is confirmed by the data [67] obtained from a study of material from disc herniations in postoperative patients, in which fragments of the endplate were found in 49% of cases.
Subchondral osteosclerosis of the spine - what is it?
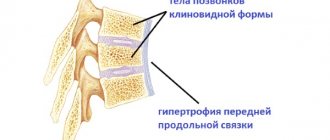
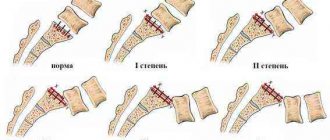
To begin with, it is worth understanding the question of how osteosclerosis of the spine develops, what it is and how it is dangerous for human health in the future. So, osteosclerosis of the vertebral bodies is a pathological process in which the lower cartilaginous layer is replaced by dense bone tissue with a discharged structure. This pathologically altered tissue does not have the protective properties of cartilage and cannot withstand increased loads. Therefore, subchondral osteosclerosis of the vertebrae, already at the initial stage of development, reveals itself by periodically occurring dull pain in the part of the spine where the lesions develop.
Subchondral osteosclerosis of the vertebral bodies is the location of foci of altered tissue on the bone surface directly under the cartilage fibers. This pathological layer further limits diffuse exchange, almost completely blocking access to periosteal resources.
Foci of sclerosing tissue can cover the entire bone surface or be localized in a limited space. Depending on this, the severity of the clinical picture varies from moderate signs to severe symptoms and rapid deterioration of the patient’s condition.
conclusions
1. The cartilaginous endplate, being a component of the intervertebral disc, performs a barrier and trophic function, providing spatial delimitation of the nucleus pulposus and vertebral bodies, as well as the entry of nutrients into the disc and the removal of catabolic products from it.
2. Degeneration of the intervertebral disc begins with changes in the cartilaginous endplate: apoptosis of chondrocytes, calcification of the cartilage matrix and blockage of the transport pathways of the disc.
3. Experimental damage to the cartilaginous endplate stimulates disc degeneration and promotes the formation of disc herniations.
4. The presence of fragments of the cartilaginous endplate in the postoperative material of patients with disc herniations confirms its role in the pathogenesis of herniation.
5. The likelihood of the presence of cartilaginous endplate material in a disc herniation increases when there is an angular defect of the vertebral body, which is one of the important potential sources of rupture-type disc herniation.
Treatment of subchondral osteosclerosis
It is necessary to begin treatment of subchondral osteosclerosis with diagnosis and elimination of all suspected causes. In our manual therapy clinic, the doctor begins to work first with the collected medical history information. The patient is given practical recommendations on changing lifestyle, diet, and distribution of physical activity during the day.
In the future, an individual course of therapy is developed for the treatment of osteosclerosis. Depending on the stage of the pathological process, the following techniques can be used:
- traction traction of the spinal column allows you to increase the space between the vertebral bodies and remove the static load from the subchondral layer;
- osteopathy and massage improve microcirculation of blood and lymphatic fluid, which accelerates the process of restoration of sclerotic tissues;
- reflexology starts the process of tissue restoration;
- Therapeutic gymnastics and kinesitherapy improve the condition of the muscular frame of the back and increase the level of protection of all tissues of the spinal column from degeneration and destruction.
Sign up for a free consultation, and our vertebrologist will give you individual recommendations for comprehensive treatment.