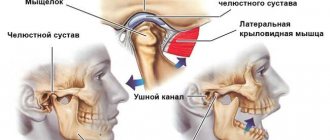
Mechanism of joint damage
The causes of joint damage in diabetes are impaired innervation and abnormally accelerated blood flow in bone tissue, leading to local osteopenia. Recurrent injuries also play a provoking role: even the most minor ones can trigger the process of osteolysis, which destroys the joint. The protein glycan composition of bone and cartilage tissue changes with insulin deficiency. The main mechanism that forms pathological disorders in bones and blood vessels is protein glycation.
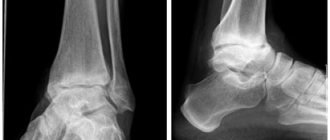
At the first stage, swelling and moderate hyperemia are observed. There is no pain or fever. X-ray examination does not reveal destructive changes. Osteoporosis is observed.
At the second stage, x-rays reveal disorders in the form of:
- moderate osteoporosis of the epiphyses;
- subchondral sclerosis, accompanied by the appearance of marginal osteophytes;
- osteolysis and sequestration;
- connective tissue growths;
- aseptic necrosis;
- pathological restructuring of bone tissue, its fragmentation.
Impaired sensitivity provokes stretching of the ligamentous apparatus, causing the joint to become loose. At the third stage, severe deformation, dislocations begin, and spontaneous bone fractures are possible. The joint literally falls apart, all that remains is to replace it with an artificial one. Mobility cannot be restored in any other way.
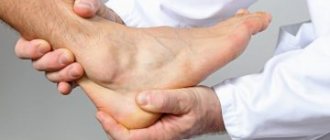
Bone pain
12978 09 June
IMPORTANT!
The information in this section cannot be used for self-diagnosis and self-treatment.
In case of pain or other exacerbation of the disease, diagnostic tests should be prescribed only by the attending physician. To make a diagnosis and properly prescribe treatment, you should contact your doctor. Bone pain: causes of occurrence, what diseases it occurs with, diagnosis and treatment methods.
Definition
Bone pain, or ossalgia, is usually a symptom of an underlying disease if it is not associated with injury - a bruise or fracture.
Types of bone pain
Bone pain can occur acutely, over a short period of time, or increase gradually and be chronic.
Subjectively, bone pain is divided into aching, twitching, throbbing, cutting, and dull.
The pain can be local or radiate (give) to other parts of the body.
Possible causes of bone pain
The most obvious cause of ossalgia is injury. As a rule, such pain is acute and intensifies with movement. Its duration depends on the extent of the injury and the progress of the recovery process.
Another reason is inflammatory bone damage, which can be a consequence of either injury or the spread of an infectious agent through the bloodstream from other foci in the body. One of the most serious diseases is osteomyelitis, which is characterized by purulent damage to bone tissue.
The risk of developing pain syndrome is present with a lack of calcium and phosphorus in the body. This condition can develop both due to insufficient intake of these microelements from food, and as a result of pathologies of the parathyroid and thyroid glands, since they are responsible for the regulation of phosphorus-calcium metabolism, as well as due to vitamin D deficiency.
Hereditary and tumor diseases that affect red bone marrow, which is located in the spongy substance of bones and bone marrow cavities and contains hematopoietic stem cells, deserve special attention.
If stem cell division is disrupted, an increase in bone marrow volume, the formation of specific metabolic products, and restructuring of the bone structure with the development of pain are possible.
In addition to tumors developing from hematopoietic cells, there are neoplasms originating from bone tissue cells (sarcoma), as well as metastases of malignant tumors of other locations.
Bone tissue often becomes the target for the development of metastatic foci, since it has a rich blood supply.
There is another cause of bone pain - the child’s “growth spurt” (the so-called night growing pains). It is associated with a sharp increase in the length of bone structures, when muscle tissue does not keep pace with bone tissue, and usually manifests itself at night.
What diseases cause bone pain?
Among the traumatic lesions leading to bone pain, the most common are fractures, cracks, and bruises.
It is worth saying that pain from dislocated joints and arthritis (inflammation of the joints) can radiate and imitate bone damage.
Among the diseases of the hematopoietic organs, namely the bone marrow, we mention leukemia (malignant tumors developing from the precursors of blood cells), thalassemia (a hereditary disease characterized by a violation of the formation of hemoglobin, a protein contained in red blood cells). A tumor of plasma cells (cells that synthesize antibodies) stands somewhat apart - myeloma, which is also characterized by bone damage with severe pain.
Osteomyelitis (inflammation of bone tissue) varies in location and causative agent, and can be acute or chronic. Late diagnosis of osteomyelitis is due to the fact that inflammation develops and spreads inside the bone, and not on the surface. Acute osteomyelitis is characterized by the occurrence of pain even when there are no external signs of inflammation.
Osteosarcoma is a malignant tumor arising from bone tissue. And the benign ones are osteochondroma, osteoblastoma, osteoid osteoma.
Cancer of the lungs, thyroid gland, and breast often metastasizes to the bones.
Hyperparathyroidism, kidney diseases leading to chronic kidney disease, diseases of the gastrointestinal tract that impair the absorption of calcium and vitamin D, lead to increased leaching of calcium from the bones and to the development of osteoporosis, characterized by the development of pain with increased stress.
Which doctors should you contact if you experience bone pain?
If pain occurs, especially acute pain, you should contact a traumatologist or surgeon. After conducting a thorough comprehensive clinical examination and additional laboratory and instrumental studies, the surgeon can refer the patient for a consultation with a hematologist, phthisiatrician,.
Diagnosis and examinations for bone pain
As a rule, the diagnosis of diseases occurring with bone pain syndrome requires visualization of the pathological focus. To do this, X-rays of the bones of the corresponding area and adjacent joints are performed.
Treatment
The basis of therapy for diabetes is constant control of sugar levels. For this purpose, there are special devices that allow you to quickly determine its level. Only if sugar is normalized can one expect results in the treatment of joints, if the degree of their destruction still allows for conservative therapy.
The patient needs to regularly do gymnastics, undergo massage and self-massage sessions, and also use hardware physiotherapy to restore sensitivity.
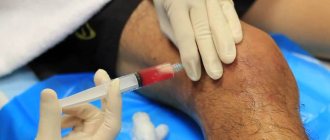
Chondroprotectors will not help with severe degenerative processes in the joint, so their use in most cases is pointless. To relieve pain, anti-inflammatory and painkillers are prescribed; with their help, swelling subsides and mobility improves slightly. Corticosteroid drugs are used by injection directly into the joint.
Lesions of the lower extremities in patients with diabetes mellitus
About the article
31589
0
Regular issues of "RMZh" No. 13 dated June 15, 2006 p. 972
Category: General articles
Authors: Volkova A.K. , Komelyagina E.Yu. , Antsiferov M.B.
For quotation:
Volkova A.K., Komelyagina E.Yu., Antsiferov M.B. Lesions of the lower extremities in patients with diabetes mellitus. RMJ. 2006;13:972.
Diabetes mellitus (DM) is one of the most common chronic diseases and is a non-infectious epidemic. Currently, about 200 million people in the world suffer from diabetes, while the number of patients increases annually by 5–7% and doubles every 15 years. According to WHO experts, their number will reach 325 million people by 2025, and 410 million people will have impaired glucose tolerance (IGT). DM is characterized by early disability and high mortality of patients due to the development of late vascular complications. This circumstance places diabetes among a number of socially significant diseases.
Lesions of the lower extremities in patients with diabetes are one of the main causes of disability, since the risk of non-traumatic amputations of the lower extremities in a patient with diabetes is 22 times higher compared to the same probability in the absence of diabetes [1]. The above fact was one of the main reasons why the St. Vincent Declaration (1989) proclaimed a 50% reduction in the number of high amputations among patients with diabetes mellitus in the world by 2000 [2]. Lesions of the lower extremities in patients with diabetes include: diabetic neuropathy (DN), lesions of the main bloodstream of the lower extremities (macroangiopathy), diabetic foot syndrome (DFS). Diabetic neuropathy Diabetic neuropathy is the presence of symptoms and/or objective signs of neuronal dysfunction in a patient with diabetes, subject to the exclusion of other causes [3]. The prevalence of the complication is quite high, depending on various examination methods, varying between 30–50% [4]. And if we add patients with subclinical forms to the number of patients with obvious clinical signs of diabetic neuropathy, then the incidence of neuropathy can reach 90% [5]. There are different classifications of diabetic polyneuropathy, one of which is given below. Classification of diabetic polyneuropathy [6]: • Hyperglycemic neuropathy • Generalized neuropathies: – sensorimotor – acute pain – autonomic – acute motor • Focal and multifocal neuropathies • Hypoglycemic neuropathy. In terms of damage to the lower extremities, sensorimotor neuropathy is most often encountered in clinical practice, which will be discussed below. Chronic hyperglycemia plays a key role in the pathogenesis of diabetic neuropathy [7]. Long-term elevated blood sugar levels lead to activation of the polyol pathway of glucose metabolism, causes oxidative stress, reduces the formation of nitric oxide, reduces blood flow in the nerve fiber, and disrupts the formation of growth factors. All of the above mechanisms cause the death of a nerve cell and lead to a slowdown in the conduction of impulses along the nerve fiber. Clinical manifestations of DN significantly reduce the quality of life of patients and are the most important pathogenetic and prognostically significant risk factor for the development of a number of complications of diabetes mellitus, in particular, diabetic foot syndrome. The clinical symptoms of DN can be positive (productive) when the patient presents certain complaints, and negative when there are no active complaints, but there are already sensory disturbances. Basically, painful and painless variants of lesions are distinguished [8]. In the absence of active complaints from the patient, the diagnosis of neuropathy can be made only on the basis of the results of examination and diagnostic testing. Among the painful forms of neuropathies, acute and chronic are distinguished [9]. The duration of the acute painful form is 6 months. Stitching, shooting, aching pains appear in the feet and legs; feeling of burning, numbness, tightening of the feet. The intensity of the pain syndrome may increase in the evening and at night. Chronic neuropathy is characterized by the presence of pain symptoms for more than six months. The clinical picture of pain varies in intensity and frequency. Compensation for diabetes can lead to regression of subjective symptoms, while inadequate control of blood glucose levels, on the contrary, worsens sensitivity and increases the feeling of numbness, burning, tingling, and paresthesia. Table 1 shows the main clinical manifestations of neuropathy depending on the stage of development of the pathological process [10]. The diagnosis of DN is established on the basis of characteristic complaints, anamnesis (duration of symptoms, presence of concomitant pathology), and objective examination data. In clinical practice, with the help of special instruments, disorders of various types of sensitivity are determined. To assess the presence of changes in tactile sensitivity, a monofilament weighing 10 g (5.07 Semmens–Weinstein) is used, pain is a prick of the back surface of the thumb with a special needle with a blunt end, temperature is by determining the difference in the sensations of heat and cold with a “tip therm” instrument, vibration - using a tuning fork or biothesiometer. Achilles and knee reflexes are used to assess motor dysfunction. For the purpose of early detection and in-depth study of DN, electromyography is performed to determine the speed of impulse transmission along the nerve fiber. The most accurate diagnostic methods include nerve biopsy followed by morphological examination. For a differential diagnosis of disturbances in the main blood flow of the lower extremities, Doppler ultrasound (USDG) of the arteries of the legs and feet is performed. Treatment of diabetic polyneuropathy Treatment of DN is characterized by an integrated approach and largely depends on the stage of development of the pathological process. Table 2 shows the principles of management of patients with diabetes mellitus and the presence of diabetic polyneuropathy. As you can see, at the initial stage of treatment, achieving compensation for carbohydrate metabolism is a prerequisite. In addition, normalization of glycemia often leads to the elimination of neuropathic pain, while drug therapy against the background of decompensation of carbohydrate metabolism may be ineffective. The prescription of symptomatic therapy is aimed primarily at eliminating the symptoms of peripheral neuropathy (pain, cramps, burning, paresthesia). The following may be prescribed: • tricyclic antidepressants (imipramine, amitriptyline) • anticonvulsants (gabapentin, carbamazepine) • analgesics • local irritants Pathogenetic drugs include: • aldose reductase inhibitors • g-linolenic acid • inhibitors of advanced glycation end products of structural proteins • drugs a- lipoic acid • nerve growth factors • myoinositol Currently, of all the above methods of pathogenetic influence, based on a number of international placebo-controlled studies, a-lipoic acid preparations have proven their effectiveness. It should be noted that one of the main objectives of managing patients with diabetic peripheral neuropathy is to prevent the development of foot ulcers. In this aspect, the main role belongs to teaching patients the rules of foot care and the selection of orthopedic shoes. Diabetic angiopathy of the lower extremities Diabetic angiopathy of the lower extremities is understood as “the presence of clinical signs such as absence of pulse in the arteries of the feet, a history of intermittent claudication, the presence of rest pain and/or changes detected during non-invasive vascular examination, indicating circulatory disorders” [3] . Reduced blood flow is the leading factor that disrupts tissue repair processes and, in combination with infection, is the main factor in lower extremity amputations [11]. The development of diabetic macroangiopathy is promoted by: smoking, arterial hypertension, chronic hyperglycemia, hyperlipidemia, age, genetic predisposition, duration of diabetes [12]. Manifestations of macroangiopathy in diabetes mellitus include: mediacalcinosis (Mönckeberg sclerosis) and atherosclerosis. Mönckeberg's sclerosis is calcification of the tunica media of the arterial wall. As a rule, mediacalcinosis in isolated form is not a cause of limb ischemia, since it is not accompanied by a decrease in the lumen of the vessel. It is believed that Mönckeberg's sclerosis is associated with autonomic polyneuropathy [13]. The course of atherosclerosis in diabetes mellitus has some differences compared to atherosclerosis in its absence [14]. Morphologically, atherosclerotic vascular lesions are characterized by a circular arrangement of plaques, systemicity, a tendency to complicated lesions with caseous decay, the formation of aneurysms, and clinically - by lesions in younger patients, the same frequency of occurrence in men and women, more rapid progression, frequent damage to the popliteal and tibial arteries. Clinically, angiopathy of the lower extremities is manifested by pain in the lower legs when walking - “intermittent claudication syndrome”, coldness of the feet. On examination - weakening or absence of pulsation of the great vessels of the lower extremities; marbled skin coloring, alopecia in the lower legs, hypothermia of the feet. In advanced cases, critical limb ischemia develops. It is characterized by the presence of ischemic pain at rest for more than 2 weeks, and/or the presence of ulcers or gangrene in the area of the foot or toes with systolic pressure in the area of the tibial arteries. To identify a decrease in the main blood flow in the arteries of the lower extremities, palpation determination is performed as a screening pulsations in the arteries of the feet. Weakening or absence of pulsation in at least one of them is an indication to refer the patient for a consultation with an angiosurgeon. The most common non-invasive method for studying the main course of the lower extremities is Doppler ultrasound (USDG) with calculation of the ankle-brachial index (ABI). ABI is the ratio of systolic blood pressure in the tibial arteries at the level of the middle third of the leg to systolic blood pressure in the brachial artery. Normally, the ABI is greater than 0.9 but less than 1.15. The degree of disturbance of the blood supply to the lower extremities is determined according to the Pokrovsky-Fontaine classification of chronic arterial insufficiency, based on the clinical manifestations of the pathology: I - functional: there are no clinical manifestations, intermittent claudication occurs during prolonged (more than 1 km) or fast walking. A decrease in ABI is detected when performing an ultrasound scan of the lower extremities. IIA – intermittent claudication when walking 500–200 m. IIB – intermittent claudication when walking less than 200 m. III – pain appears at rest. IV – presence of ulcerative-necrotic lesions of the lower extremities. However, it should be remembered that in patients with severe polyneuropathy, accompanied by decreased sensitivity, this classification does not always objectively reflect the severity of ischemia. The patient may not feel pain, which leads to underestimation of the severity of the condition. It is also necessary to know that the determination of ABI in the presence of mediacalcinosis is accompanied by falsely high ABI values (>1.15). Advanced methods for diagnosing lesions of the main blood flow of the lower extremities include duplex scanning of the arteries. Angiography is considered the “gold standard” for studying the vascular bed. The indication for performing angiography or aorto-arteriography is the identification of stenosis or occlusion by ultrasound to determine the possibility of performing reconstructive surgery. To assess the severity of tissue ischemia, the method of determining transcutaneous oxygen tension in the lower extremities is becoming increasingly widespread. Through transcutaneous gas analysis, it is possible to measure local oxygenation (TspO2) of the skin and, thus, assess the prognosis of healing of the wound defect. The level of TcpO2 is considered a critical indicator. Comprehensive management of patients with diabetes mellitus with impaired main blood flow involves addressing the following issues [16]: 1) improving the main blood flow; 2) normalization of carbohydrate metabolism (HbA1c 3) correction of blood pressure (target parameters should be considered blood pressure 4) prescription of drugs that reduce the increased thrombogenic potential of the blood (taking acetylsalicylic acid in a dosage of 50–325 mg is mandatory); 5) correction of the blood lipid spectrum (total cholesterol 1.2 mmol/l, triglycerides 6) adherence to a dosed walking regimen; 7) quitting smoking. To resolve issues of improving the main blood flow, patients with diabetes mellitus must be managed together with an angiosurgeon. The most effective method is reconstructive surgical interventions on blood vessels: bypass surgery (aorto-femoral, femoral-popliteal, femoral-tibial), percutaneous transluminal balloon angioplasty (alone or in combination with the installation of an endovascular stent). To improve the rheological properties of blood, vasoactive drugs are prescribed. In addition to platelet disaggregants (acetylsalicylic acid), the use of which is currently considered mandatory in this category of patients, drugs with complex effects are prescribed (pentoxifylline 1200 mg per day). Recently, reports on the beneficial effect of Tanakan (EGb 761) at 120 mg/day have been published quite often. at least 3 months. The effectiveness and safety of Tanakan for obliterating atherosclerosis of the lower extremities has been proven in a number of international double-blind placebo-controlled studies, which recorded a significant increase in walking distance before the onset of pain [17–19]. The beneficial vasoactive effect of Tanakan was objectified using Doppler ultrasound of the lower extremities [20]. We must not forget that uncontrolled use of vasoactive drugs can lead to the progression of retinopathy (hemorrhage into the vitreous body and retina). Therefore, throughout the prescription of vasoactive drugs, dynamic observation by an ophthalmologist is necessary. It should be noted that due to the presence of hemorheological, metabolic, neuroprotective and antioxidant effects in Tanakan’s mechanism of action, the drug has a protective effect on the retina. The safety and effectiveness of Tanakan in the treatment of patients with diabetic retinopathy was also confirmed by both foreign and Russian specialists during experimental and clinical studies [21]. The absence of contraindications, drug-drug interactions and the favorable side effect profile of Tanakan identified in these studies allows us to recommend its wider use in elderly patients with diabetes mellitus when they have a combination of polyneuropathy, angiopathy and retinopathy. Diabetic foot Diabetic foot (diabetic foot syndrome - DFS) is an infection, ulcer and/or destruction of deep tissues associated with neurological disorders and/or a decrease in the main blood flow in the arteries of the lower extremities of varying severity [11]. Manifestations of DFS include “painless progressive destruction of one or more joints of the foot against the background of neuropathy - diabetic osteoarthropathy (Charcot foot) [22].” DBS is one of the leading disabling chronic complications of diabetes mellitus. From 40 to 60% (in some regions up to 90%) of all non-traumatic amputations are performed in patients with diabetes mellitus. In 85% of cases, all amputations associated with diabetes mellitus are preceded by infected foot ulcers, the prevalence of which is 4–10% among diabetic patients over 50 years of age. In most cases, amputations are performed due to a combination of deep tissue infection and ischemia: in 50-70% of cases, the cause of amputations is gangrene, in 20-50% - infection. It is necessary to distinguish between risk factors for diabetic foot syndrome and risk factors for amputation. As a rule, a peptic ulcer occurs as a result of a combined interaction of several risk factors. Risk factors for the development of diabetic foot syndrome, according to leading experts, can be presented as follows: peripheral neuropathy; peripheral vascular pathology; previous ulcers and/or amputations; foot deformities; poorly chosen shoes; injury; socio-economic factors; race [23,24]. Risk factors for amputation include: infected foot ulcers; infected foot ulcers in combination with ischemia. Currently, there is no single classification that takes into account all the features of the etiopathogenesis of diabetic foot syndrome. The following classifications have been proposed, taking into account various factors in the formation of this complication, such as: 1) the form of the lesion. Depending on the predominance of peripheral neuropathy or macroangiopathy, the following are distinguished: – neuropathic, – ischemic (in isolated form it is extremely rare), – neuro-ischemic forms of diabetic foot syndrome [11]. 2) depth of spread of the ulcer (Wagner classification) [25]. Depending on the depth of the lesion, the ulcerative defect is assigned a grade from 0 to V: Art. 0 – intact undamaged skin st. I – superficial ulcer (the process involves the epidermis, dermis) st. II – the process involves the skin, subcutaneous tissue, soft tissues. III – deep ulcer, abscess, osteomyelitis, septic arthritis Art. IV – dry/wet gangrene: necrosis of all layers of skin in individual areas of the foot, Art. V - dry/wet gangrene of part of the foot/all foot 3) the severity of the infectious process: • the infection that does not threaten limbs, characterized by the presence of signs of inflammation. Soft tissue edema, hyperemia and hyperthermia spread to less than 2 cm. As a rule, it is superficially located ulcerative defects that do not require hospitalization. • The infection threatening limbs. Soft tissue edema, hyperemia, hyperthermia spread by more than 2 cm, capturing deeply lying structures (subcutaneously fatty fiber, fascia, joints, bones) with the presence of necrotic and purulent discharge (plantar abscess, septic arthritis, feet of the foot, osteomyelitis). In this case, hospitalization is required for the relevant therapeutic measures. The main manifestation of the SDS is trophic ulcers or other purulent -destructive lesions of the foot. It should be noted that the ulcer of the lower leg, developing against the background of chronic venous failure, do not belong to the definition of SDS. Table 3 shows differential diagnosis of neuropathic and neuroichemic ulcers for SDS. The basic principles for the treatment of ulcerative defects in patients with diabetes in patients with diabetes strategy of comprehensive treatment of neuropathic infected ulcers consists of the following basic provisions: 1) surgical treatment of the purulent focus (the volume of intervention is determined by the severity of the existing infectious process); 2) the appointment of systemic rational antibacterial therapy; 3) local treatment of wounds using multicomponent ointments on a hyperosmolar basis, antiseptics and dressings that contribute to the purification of the wound; 4) execution of the unloading mode (fasting heel, use when walking crutches/ seats - strollers, etc.); 5) plastic and reconstructive organ -preserving operations. With the neuroichemic version of the lesion, the principles of therapeutic measures are as follows: 1) correction of arterial blood flow in the lower extremities (surgical and conservative); 2) surgical treatment of a purulent -non -chorotic focus (the volume of intervention is determined by the severity of the existing infectious process and compensation for arterial blood flow); 3) the appointment of systemic rational antibacterial therapy; 4) local treatment of wounds using solutions of iodophores, antiseptics and dressings that contribute to the purification of the wound; 5) if necessary, performing the unloading mode (unloading heel, use of crutches/seats - inks when walking, etc.); 6) plastic and reconstructive organ -preserving operations. Due to the fact that with diabetes, the risk of gangrene of the foot and, as a result, amputation of the limb, each patient should be familiar with the complex of preventive measures that allow us to sufficiently reduce this risk. Patients cannot be: trim the nails on their feet with scissors or use nippers; use corn -layers; walk barefoot; wear high -heeled shoes and a narrow nose; wear "slaps"; Heat your legs next to the heat source (next to the fireplace or on the battery). At the same time, patients need to: regularly file nails with a file; every day wash your legs in warm water, thoroughly wiping interdigital spaces after washing; Buy new shoes in the afternoon, when the legs were already slightly swollen; wear only cotton socks (warm your legs in socks from wool); wear only comfortable low -heeled shoes; With any wound on the legs, immediately contact the office of a diabetic foot. Compliance with these simple rules will significantly reduce the likelihood of amputation of the limb. Literature 1. Trautner C., Haastert B., et al. Amputations and diabetes: a case - comol Study // Diabet Med. - 2002. - Vol. 19. - P. 35–40. 2. Diabetes Care and Research in Europe: The St.vinsent Declaration. Geneva: World Health Organization. ICP/CLR 034 // - 1989. 3. International agreement on the diabetic foot, 2000. 4. Kempler P. Neuropathies - Springer - Budapest - 2002; Dyck Pj, Litchy Wj The Rochester Diabetic Neuropathy Study of Health Subjects. // Neurology, 1995, 45: 1115–1121. 5. Vinik AI, Liuzze Fi, ETS. Diabetes Neuropathy. Diabetes Care, 1992, 15: 1926–1975. 6. Thomas PK “Nextbook of Diabetic Neuropathy”, 2002. 7. The Diabetes Control and Complications Trial Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long - Term Complication in -Depness Diabetes Mellitus n.engl. J.Med. 1993; 70: 1009–1018. 8. International guidelines on the out -Patient Management of Diabetic Peripheral Neuropathy. Abingdon: The Medicine Group (Education) Ltd., 1998. 9. Kempler P. Neuropathies. Springer. Budapest. 2002. BULTON AJM “Nextbook of Diabetic Neuropathy”, 2002. 11. International agreement on diabetichek stop. - M., 1999. 12. Grandfather I.I., Antsiferov M.B. and co -authors. Diabetic foot syndrome. - M., 1998. 13. Young M. et al., Medial Arterial Calcification in the Feet of Diabetic Patients and Matched Non -Diabetic Control Subjects // Diabetologia. - 1993. –VOL.36. - P. 615–621. 14. Jude E., et al., Peripheral Arterial Disease in Diabetic and Nondiabetic Patients // Diabetes Care. - 2001. - Vol. 24. - P. 1433–1437. 15. Luther M. Critical Limb Ischemia in Diabetes // VASA. - 2001. - SUPPL. 58. - P. 21–27. 16. ADA: Clinical Practice Recommentations 2004 // Diabetes Care 2004; 27: S19 - S75. 17. Bauer U. etude clinicue d i/ egb 761 dans 1'artertedes Mambers inferieurs. Essai a duble Insu Face au Placebosur 6 Mois. // Arzneim Forsh Drug Res. –1984. –V.34. –N.6. –P.716–720. 18. Koshkin V.M. Conservative therapy of chronic obliterating diseases of the arteries of the limbs. // Russian Medzinsky Journal. –1997. –T.6. - No. 13. —s.820. 19. Pokrovsky A.V., Chupin A.V., Gryaznov O.G. The clinical test of the drug Tanakan in patents with atherosclerotic damage to the arteries of the lower extremities. // International Symposium “Consecious therapy in angology. Experience in the use of Tanakan and Gingko Fort preparations. ” - M. –1997. - Abstracts. - S.37. 20. Aliyev M.A., Dzhakupov V.A., Sekerbaev O.A. Conservative treatment of diabetic angiopathies. // in Sat: Surgical treatment of diabetic angiopathies. - Almaty. –1998. —P.29–32. 21. Moshetova L.K., Arzhimatova G.Sh., Strokov I.A., Yarovaya G.A. "Modern antioxidant therapy of diabetic retinopathy." Russian medical journal, 2006, v. 7, No. 1, 26–38. 22. Frykberg RG “Charcot Foot: An Update on Pathogenesis and Management” in the Foot in Diabetes, 2000, P.236. 23. Peter - Riesch b et al.: Pivotal Events: A Neglected Field of Factors Leading to Major Diabetic Foot Complications. // Diabetologia. 1996; 39, SUPPL. 1, A.265. 24. Veves A., et all: Comparison of Risk Factors for Foot Problems in Diabetic Ptions Attending Teaching Hospital Outpatient Clinics in Four Different European States. // Diabetic medicine. 1994; 11: 709–711. 25. Wagner FM a classification and Treatment Program for Diabetic, Neuropatic and Dysvascular Foot Problems. In the American Acaemy of Ortopaedic Surgeons Instructions Lectures. - St. Louis: Mosby, 1979. - P. 143–165.
Content is licensed under a Creative Commons Attribution 4.0 International License.
Share the article on social networks
Recommend the article to your colleagues
Surgery
Surgery for diabetes mellitus is possible only if sugar levels are normalized. If diabetes is compensated and no contraindications from the cardiovascular system or internal organs are identified, then joint replacement can be performed. The endocrinologist gives a conclusion based on the examination results. When sugar levels are normalized, all healing processes in diabetic patients occur in the same way as in healthy people, but control is very important not only before surgery, but also immediately after it.
There is a risk of rejection if the patient is old and the disease has lasted for more than 10 years. However, modern technologies make it possible to reduce it to a minimum. Endoprosthetics are performed using cement that contains an antibiotic (for example, cifuroxime). The incidence of complications with the introduction of this method was significantly reduced.
Excess weight, which often exceeds all norms for diabetes mellitus, significantly complicates the endoprosthetics procedure. This may be a contraindication to surgery, since not only does access to the joint, ultrasound navigation and subsequent tissue closure become difficult, but also survival rate deteriorates due to the high load. There are frequent cases of early infections and displacement of the endoprosthesis; there is a high probability that a periprosthetic fracture will occur, joint ligaments will be damaged, limb thrombosis, thromboembolism, aseptic instability, and dislocation of the implant head may develop.
The success of a joint replacement operation for an obese person diagnosed with diabetes mellitus largely depends on the doctor’s experience, the availability of the necessary equipment for accurate installation of the endoprosthesis, and the correct selection of the implant. It is important to carry out competent preoperative prevention of thrombosis and infection.
There are clinics in Russia that can provide all the necessary conditions for the most successful endoprosthetics of such patients, but getting there is often difficult. The only opportunity to replace a joint with minimal risk of complications is to travel to Germany or Israel. But many are stopped by the high cost. proposes to organize treatment of the musculoskeletal system for patients from the Russian Federation in specialized clinics in the Czech Republic, where prices are significantly lower and quality is at a high European level.
Osteoarthritis (OA) and type 2 diabetes mellitus (DM2) are among the most common diseases among elderly and senile people, and therefore the combination of these diseases is widespread among people in this category [1, 5]. Diabetes introduces into the clinical picture of OA a greater severity of degeneration of cartilage tissue, a distinct periarticular inflammatory process and a decrease in the performance of the thigh muscles, which is mainly associated with the development of late diabetic complications (macro- and microangiopathy, sensorimotor neuropathy) [1, 3, 4 , 6, 8—10, 12]. However, a number of studies have revealed that chronic hyperglycemia prevents the development of secondary inflammatory reactions in the joint cavity, which may manifest itself as less severe pain and limited range of motion in the knee joint in patients with type 2 diabetes compared to individuals suffering from gonarthrosis without disorders of carbohydrate metabolism [2— 4]. Literature data on the frequency and severity of articular syndrome depending on the duration and degree of compensation for T2DM are contradictory. A number of authors note the presence of deeper osteoarticular disorders in patients with long-term, poorly controlled diabetes. At the same time, other researchers believe that severe articular syndrome is often found among people with mild T2DM [3, 13]. The purpose of this work was to study the clinical and metabolic aspects of the influence of T2DM on the course of gonarthrosis.
Material and methods
A comprehensive examination of 90 people aged from 40 to 70 years was carried out. The 1st (main) group included 45 patients with gonarthrosis in combination with T2DM. The 2nd (control) group included 45 patients with gonarthrosis without concomitant T2DM. In both groups, women predominated among patients (90%). The criteria for inclusion of patients in the groups were the presence of gonarthrosis (according to the criteria of R. Altman [7]) and the intensity of pain when walking was at least 40 mm on a visual analogue scale (VAS). Patients with type 1 diabetes and other specific types of diabetes, cancer, and those with radiographic stage IV OA were excluded from the study.
The examination of the joints included examination, palpation, measurement of the circumference of the knee joints, objective assessment of pain at rest and when walking using VAS, and goniometry of the knee joints. Symptoms of gonarthrosis were also assessed by the severity of pain and functional Lequesne index in accordance with a specialized questionnaire [11]. X-rays of the knee joints were taken, and the stages of gonarthrosis were established in accordance with the classification of I. Kellgren and J. Lawrens (1957). The diagnosis of T2DM was established in accordance with the diagnostic criteria proposed by the WHO Expert Committee (1999). The degree of diabetes compensation was assessed by the level of glycated hemoglobin (HbA1c).
Statistical processing was performed using Statistica 6.0 software packages (StatSoft Inc., USA). Absolute values are presented as median and quartiles. When conducting multiple comparisons, Kruskal-Wallis rank analysis of variance was used to assess the significance of differences in means; pairwise comparisons were performed using the nonparametric Mann–Whitney test. To assess the significance of differences in proportions, the Pearson chi-square test was used. Differences were considered significant at a significance level of p<0.05.
Results and discussion
Clinical characteristics of the compared groups are presented in table. 1.
It should be emphasized that the study and control groups were comparable in age, gender, duration and stage of gonarthrosis, and basic morphometric parameters, including body mass index (BMI).
Features of articular syndrome with combined pathology include greater severity of pain during movement (Table 2).
The Lequesne functional test confirmed the greater severity of gonarthrosis according to the total points in the 1st group of patients. The circumference of the knee joints clearly prevailed in the group of patients with combined pathology. Goniometry revealed a decrease in the angle of flexion and extension of the maximally affected joint in group 1 of patients, which also indicated a dysfunction of the joint.
Lequesne's test was analyzed according to the frequency of occurrence of each sign in groups of patients. According to this test, 27% of patients in group 1 and 9% of patients in group 2 experienced night pain at rest (p=0.01). Patients in group 1 were characterized by increased pain in the knee joints after standing for 30 minutes; 65% of patients in group 1 indicated this sign compared to 31% of patients in group 2 (p=0.001). An analysis of the maximum distance covered without pain showed that only 11% of patients in the group of combined pathologies can walk a distance of 1 km or more without pain. In the control group, this figure was 51% (p=0.02). The appearance of pain when walking at a distance of less than 300 m was significantly more often registered among patients of group 1 - 28%, versus 11% of patients in the control group (p = 0.01). The data indicate a worsening of the joint syndrome against the background of T2DM (increased pain and more pronounced limitation of joint function).
To assess the effect of the duration of T2DM on the course of gonarthrosis, patients with concomitant pathology were divided into 3 subgroups and all characteristics of gonarthrosis were compared (Table 3).
Impaired joint function, assessed by the Lequesne index and a decrease in the angle of flexion of the knee joints, and gonarthrosis, predominantly of X-ray stage III, were statistically significantly more often determined in patients with a duration of T2DM of more than 10 years. Secondary synovitis was more common in patients with OA in combination with T2DM lasting less than 5 years. Patients in this subgroup significantly more often complained of night and starting pain. This indicates the predominance of the inflammatory component in this group of patients.
In addition to the duration of T2DM, the degree of compensation for T2DM had a significant impact on the course of the articular syndrome (Table 4).
An increase in pain intensity according to VAS, joint dysfunction according to the Lequesne index, X-ray stage III of gonarthrosis, the presence of morning stiffness and secondary synovitis, complaints of night and starting pain were more often determined with severe decompensation of T2DM, characterized by an HbA1c level>10%.
conclusions
1. T2DM aggravates the course of OA in patients with concomitant pathology. The presence of T2DM in a patient with gonarthrosis leads to increased pain, deterioration of joint function, and a higher incidence of secondary synovitis.
2. Severe decompensation of carbohydrate metabolism (HbA1c>10%) is associated with increased pain (more than 80 mm on VAS), more pronounced functional disorders and inflammatory changes in the joints in patients with gonarthrosis in combination with T2DM.